Rapid, mass decontamination following a nuclear reactor accident or terrorist “dirty bomb” attack may be in the offing with Berkeley Lab researchers reporting they have begun seeking FDA approval for trials of an orally administered decontamination agent.
The process of decontaminating the body of radioactive particles hasn’t advanced much in half a century, with only one chemical agent available. That compound, known as DTPA, is widely regarded as a relic from the Cold War and is only partially effective at removing radioactive elements from the body.
The lack of research in the area is somewhat surprising given that Japanese officials considered the evacuation of more than 30 million Tokyo residents following last year’s triple meltdown at the Fukushima nuclear reactor.
Now, however, Berkeley Lab scientists say they have developed a much more effective treatment that decontaminates a large number of the elements (known as actinides) that are likely to be part of the radiation exposure from a nuclear plant or weapon, including plutonium, americium, curium, uranium and neptunium.
Depending on the level of radiation exposure and how soon treatment can start, the researchers claim one of these pills would result in the excretion of approximately 90-percent of the actinide contaminants within 24 hours. Taking one pill daily for two weeks should, they believe, be enough to remove virtually all of the actinide contaminants.
“With the expanding use of nuclear power and unfortunate possibility of nuclear weapon use, there is an urgent need to develop and implement an improved therapy for actinide contamination of a large population,” says Berkeley Lab scientist Rebecca Abergel. “We are now in the process of demonstrating that our actinide-specific decontaminating agents are ready for clinical development.”
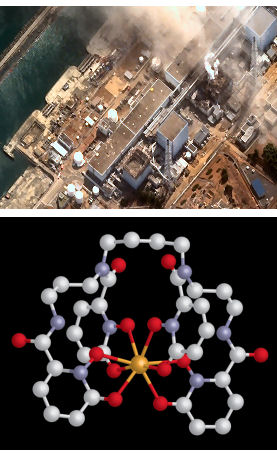
“Since the biochemical properties of plutonium and iron are similar, we modeled our sequestering agents after the chelating unit found in siderophores,” explained the research leader Ken Raymond. Siderophores are small molecules secreted by bacteria to extract and solubilize iron.
The technique of copying Mother Nature appears to have paid dividends, with Raymond claiming the sequestering agents (known as hydroxypyridonate ligands, or HOPOs) his team have created are unrivaled in terms of actinide-affinity, selectivity and efficiency.
The two best candidate HOPOs developed by the team are a tetradentate, which has four chelating arms, and an octadentate (pictured), which has eight chelating arms. The “arms” in this case are atoms with pairs of electrons available for covalent bonding with an actinide.
Each variant has its own merits, explains Abergel. “A single octadentate HOPO can form a full actinide complex and results in more total actinide excretion. However, it is easier for the smaller tetradentate HOPO to pass through biological membranes and access desired target sites in the body. Both warrant further development for emergency use in the case of a radiological event.”
Abergel says the team have carried out extensive experiments in animal models and human cell lines that have established the two HOPO candidates as being highly effective and non-toxic at the tested doses. She and her group have now started the process with the FDA to determine what further data is needed to move into clinical trials.
Related:
Discuss this article in our forum
Take Two Rads And Call Me In The Morning
Radiation exposure impacting gender ratio
Radiation’s bad rep a beat-up, controversial new research claims
Radiation Resistant Organism Reveals Its Secrets

















Comments are closed.