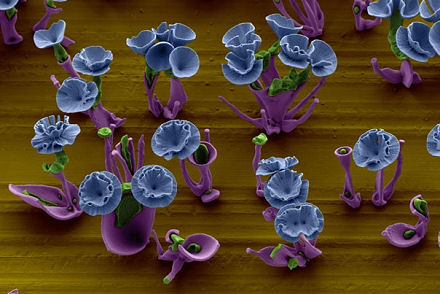
By simply manipulating chemical gradients in a beaker of fluid, researchers at Harvard have found they can control the growth behavior of crystals to create beautiful, precisely tailored nanostructures.
“For at least 200 years, people have been intrigued by how complex shapes could have evolved in nature. This work helps to demonstrate what’s possible just through environmental, chemical changes,” said lead researcher Wim L. Noorduin.
The precipitation of the crystals depends on a reaction of compounds that are diffusing through a liquid solution. The crystals grow toward or away from certain chemical gradients as the pH of the reaction shifts back and forth. The conditions of the reaction dictate whether the structure resembles broad, radiating leaves, a thin stem, or a rosette of petals.
It is not unusual for chemical gradients to influence growth in nature; for example, delicately curved marine shells form from calcium carbonate under water, and gradients of signaling molecules in a human embryo help set up the plan for the body. Likewise, bacteria living in colonies can sense and react to plumes of chemicals from one another, which causes them to grow, as a colony, into intricate geometric patterns.
Replicating this type of effect in the laboratory was a matter of identifying a suitable chemical reaction and testing, again and again, how variables like the pH, temperature, and exposure to air might affect the nanoscale structures.

“You can really collaborate with the self-assembly process,” explains Noorduin. “The precipitation happens spontaneously, but if you want to change something then you can just manipulate the conditions of the reaction and sculpt the forms while they’re growing.” Increasing the concentration of carbon dioxide, for instance, helps to create ‘broad-leafed’ structures. Reversing the pH gradient at the right moment can create curved, ruffled structures.
“Our approach is to study biological systems, to think what they can do that we can’t, and then to use these approaches to optimize existing technologies or create new ones,” said co-researcher Joanna Aizenberg. “Our vision really is to build as organisms do.”
Related:
Discuss this article in our forum
Evolutionary Algorithms Used To Design A Better Leaf
Bacteria used to crank-up tiny machines
Molecular “robot” built from DNA
Battery built from GM viruses





Comments are closed.