I am going to take a chance and reply to the dummies link
you provided , hopefully you can answer my question about the
wording on the page and its assoiated image.
please dont include the history of QM as my question does not require a full history , only a simple answer.
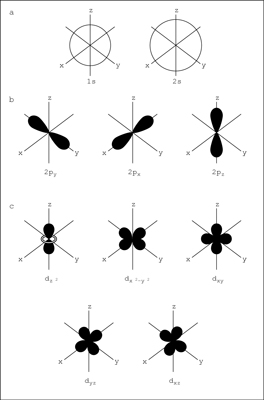
As shown in the top row of the figure (a), there are two s orbitals — one for energy level 1 (1s) and the other for energy level 2 (2s). The s orbitals are spherical with the nucleus at the center. Notice that the 2s orbital is larger in diameter than the 1s orbital. In large atoms, the 1s orbital is nestled inside the 2s, just like the 2p is nestled inside the 3p.
I can fully understand (a) in the above image of a QM model.
moving on to (b) I get lost
there is no 3p shown!
would the 3p if shown only represent a smaller orbital?
plus: the web page did not make me really understand the model.
the only thing that the web page showed me was that the electron orbits in the same way as it always has.
the 2p..3p ... and so on are only probability orbitals , not electron orbitals , and are only used to estimate where electrons
might be as they orbit in 1s ...2s ....3s ... etc using mathematics.
maybe that's why they use ( p ) for probability.
if QM is just a means of estimation that can be utilize in actual equipment used to carry out experiments then it cannot claim that
the bohr atom is incorrect as a atom model.
the bohr atom model would be an incorrect model of a QM probability model however.
using QM math to trigger a strobe light is probably how
actual scientist see electrons as they orbit in s1..s2..s3 orbitals.
but I think it is safe to say that actual electrons in orbit around an actual nucleus do not follow the orbital paths
p , d shown in the QM atom model.




