|
0 members (),
196
guests, and
2
robots. |
|
Key:
Admin,
Global Mod,
Mod
|
|
|
|
Joined: May 2011
Posts: 2,819
Megastar
|

Megastar
Joined: May 2011
Posts: 2,819 |
Yes Bill you are correct and I understand what you are saying now thank you for that clarification. I expressed in another thread once that the whole model of atoms as something like little planetary systems seems so bizarre, most realized back then it was wrong and yet such a silly concept has gained such acceptance with the public. I once did an excercise to try and work it out because it was suspected it was wrong in 1913 they knew it was definitely wrong in 1917 and by the time Pauli had finished his work in 1925 it was dead and buried and yet it survived and haunts us today. Disney even made films about this rubbish and cemented the idea with the public and so today we have people still convinced atoms look like bohr's crazy atom. I had to laugh even if you go to the dummies guide http://www.dummies.com/how-to/content/atomic-structure-the-bohr-model.html
Although the Bohr model is still used today, especially in elementary textbooks, a more sophisticated (and complex) model — the quantum mechanical model — is used much more frequently.
No kidding because the Bohr model is garbage thats is why we use the quantum model so why has the Bohr model not been buried and given it's last rites. It's not that hard to teach the quantum model and usually it makes a hell of a lot more sense once the students understand it. My only answer I can come up with is the word Quantum scares people because apparently Quantum Mechanics is a scary thing. Oh I should say dummies does do a good job on the quantum atomic model ... see not that scary http://www.dummies.com/how-to/content/atomic-structure-the-quantum-mechanical-model.htmlI note even our mate Socratus noted there is something wrong with Bohr's model as per his ABC on QM book quote ... I wonder if he read on further and understand how QM solves the problem and even why QM can explain why it will only react with certain frequencies. I should also say this is my favourite image of an atom because for people it makes it clear the idea of the electrons being not interconnected to the nucleus is removed which to me is the biggest misleading part of the rutherford and bohr models. 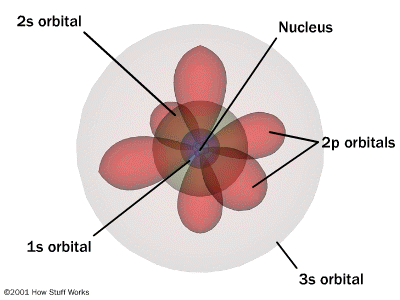
Last edited by Orac; 09/06/12 04:53 PM.
I believe in "Evil, Bad, Ungodly fantasy science and maths", so I am undoubtedly wrong to you.
|
|
|
|
|
Joined: Dec 2010
Posts: 1,858
Megastar
|

Megastar
Joined: Dec 2010
Posts: 1,858 |
also , you registered in 2010 , thats only 2 years , so how could I have been waving my hands and claiming that your wrong for many many years when you havent even been here for many many years? I have only been registered for 2 years. I have been lurking for many many years. I finally registered when somebody said something so ridiculous that I felt I had to respond. So I can recall many many times when you have done your hand waving attempting to convince people that you are right and the rest of the world is wrong. Bill Gill
C is not the speed of light in a vacuum.
C is the universal speed limit.
|
|
|
|
|
Joined: Mar 2006
Posts: 4,136
Megastar
|

Megastar
Joined: Mar 2006
Posts: 4,136 |
I have only been registered for 2 years. I have been lurking for many many years. I finally registered when somebody said something so ridiculous that I felt I had to respond. So I can recall many many times when you have done your hand waving attempting to convince people that you are right and the rest of the world is wrong.
Bill Gill then perhaps you should have clarified that in your post. Of course he is doing a lot of hand waving to claim I am wrong, but that is what he has been doing for many many years. my waving my hand is not as good as the rest of the world waving their hand? you call it waving your hand as if to taunt me , am I wrong about that? am I waving my hand when I ask that question? is there another sentence you could have used that would not have caused me to think that your choice of words was a taunt? its as if you are trying to extract an emotional response from me.
3/4 inch of dust build up on the moon in 4.527 billion years,LOL and QM is fantasy science.
|
|
|
|
|
Joined: Aug 2010
Posts: 3,570
Megastar
|

Megastar
Joined: Aug 2010
Posts: 3,570 |
its really no big deal Bill s I agree completely. It just seems a shame that so many non-science things appear to become big deals.
There never was nothing.
|
|
|
|
|
Joined: Mar 2006
Posts: 4,136
Megastar
|

Megastar
Joined: Mar 2006
Posts: 4,136 |
I know that I hit the reply button on your post orac , but Im replying to the image you posted , not anything that you included as text in your post condemning the bohr atom.  if the above is a model of an atom , and the electron (or whatever quantum mechanics has converted it into) is probably somewhere in the universe at some point in time , or somewhere in the vicinity of the nucleus traveling inside the red balloon looking orbital things pictured above. 1) the electrons charge must be changing levels almost as fast as the speed of light. 2) what causes the energy level changes that obviously must be occurring? 3) are there no energy levels anymore in QM? 4) are there still electrons in QM? 5) does the electron actually orbit the nucleus or does it pass through the nucleus , or does it get close to the nucleus then fly away because of some QM effect? Im just curious , I welcome anyone to reply , but please keep it civil.
3/4 inch of dust build up on the moon in 4.527 billion years,LOL and QM is fantasy science.
|
|
|
|
|
Joined: Mar 2006
Posts: 4,136
Megastar
|

Megastar
Joined: Mar 2006
Posts: 4,136 |
I agree completely. It just seems a shame that so many non-science things appear to become big deals. I agree also , I think its mainly just filler. but it does turn out to have a snowball effect. so , lets agree to agree that we agree to end this. or at least that we agree to end this , do you agree? LOL
3/4 inch of dust build up on the moon in 4.527 billion years,LOL and QM is fantasy science.
|
|
|
|
|
Joined: Dec 2006
Posts: 962
Superstar
|

Superstar
Joined: Dec 2006
Posts: 962 |
Maybe he is measuring time in internet years. An internet year can seem like ten years, so 2 years can seem like 20 subjectively. :-)
If you don't care for reality, just wait a while; another will be along shortly. --A Rose
|
|
|
|
|
Joined: Mar 2006
Posts: 4,136
Megastar
|

Megastar
Joined: Mar 2006
Posts: 4,136 |
I think I first registered on SAGG in the summer of 1999 , that was back when Amaranth Rose I was on the forum.
it does seem like a long time ago even though it was only 13 years.
I show up as registering in 2006 now I think because the site
dropped it's database , remember that?
it happened several times.
3/4 inch of dust build up on the moon in 4.527 billion years,LOL and QM is fantasy science.
|
|
|
|
|
Joined: Dec 2010
Posts: 1,858
Megastar
|

Megastar
Joined: Dec 2010
Posts: 1,858 |
Drat, I lost the post I was trying to put together.
I used to post fairly regularly back before SAGG started requiring registration to post. I kept looking in regularly even though I wasn't posting. Then as I said above I registered to respond to a particularly bad post.
Bill Gill
C is not the speed of light in a vacuum.
C is the universal speed limit.
|
|
|
|
|
Joined: May 2011
Posts: 2,819
Megastar
|

Megastar
Joined: May 2011
Posts: 2,819 |
Im just curious , I welcome anyone to reply , but please keep it civil.
Paul I have always been willing to civil so long as things don't become a troll excercise. I am usually quite explicit about what science consensus believes and what I believe I do have some discrepencies with science consensus like all scientists do but I can't prove my beliefs so they are unproven ideas of mine. Really understanding the model is extremely easy the link provided does it. http://www.dummies.com/how-to/content/atomic-structure-the-quantum-mechanical-model.htmlWhat is important here is not whether you or I believe it but that experimentation proves it to be the fact. Again science is not a popularity vote and noone has been able to prove the model wrong. Infact only this week for example now that getting temeprature near absolute zero has become mundane thanks to laser cooling scientists worked out they could seperate gases based on there quantum spin property of the model http://phys.org/news/2012-08-one-way-street-atoms.htmland we now even watch the transistions between classic phases of matter http://phys.org/news/2012-09-ultracold-atoms-reveal-quantum-effects.htmlSo again it's not up to me to convince you the Bohr model is wrong science has already done that and thrown it in the bin. If you want the Bohr model back at science you must explain each and everyone of the thousands of experiments that currently prove substaintive proof of the quantum model you can't just wave hands at it and say I don't know but I don't like it. Einstein disliked Quantum Mechanics and as good a scientist as he was we didn't let him do it either. So science has assigned the Bohr model to the bin the reality is that it should have been done so in 1925 and it certainly should not be appearing in current text books. For me the reason it does because of past historic disquiet over QM from great scientists like Einstein. People trusted Einstein in this case he was wrong and in someway that legacy will take time to eliminate. I did my early science years as an Einstein GR/SR junkie and disliked QM. However as experiment after experiment result rolled in throught the early 2000's you could see that QM was not only correct and not going to go away or be explained away. Now I study QM and it is a most interesting area because it is moving so fast and getting faster because with QM understanding has come the ability to simplify the equipment. Laser cooling has change the landscape in getting down to absolute zero the enormous cryogenic labs of the past which limited which universities had the ability to do research have gone. QM experimentation is now within the reach of any university and thus the field has exploded.
Last edited by Orac; 09/07/12 03:00 AM.
I believe in "Evil, Bad, Ungodly fantasy science and maths", so I am undoubtedly wrong to you.
|
|
|
|
|
Joined: Aug 2010
Posts: 3,570
Megastar
|

Megastar
Joined: Aug 2010
Posts: 3,570 |
why has the Bohr model not been buried and given it's last rites. Looking back to the '70s and early 80s when I was trying to explain very basic mineralogy to young people, many of whom had a distinctly limited learning ability, the Bohr model was an ideal tool for getting the idea over. It was all that was needed for the task. You could then feel as though you were doing your bit for science by adding something like: "it's not really as simple as that, but it gives a reasonable picture of how the chemistry works". I suppose I, and people like me, have to take some blame for keeping the Bohr model "kicking", if not actually alive. 
There never was nothing.
|
|
|
|
|
Joined: Mar 2006
Posts: 4,136
Megastar
|

Megastar
Joined: Mar 2006
Posts: 4,136 |
I am going to take a chance and reply to the dummies link you provided , hopefully you can answer my question about the wording on the page and its assoiated image. please dont include the history of QM as my question does not require a full history , only a simple answer. 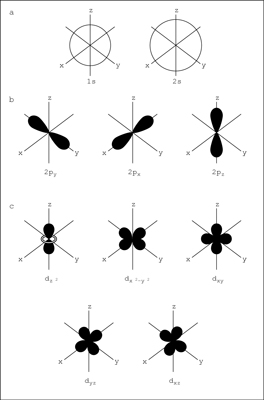 As shown in the top row of the figure (a), there are two s orbitals — one for energy level 1 (1s) and the other for energy level 2 (2s). The s orbitals are spherical with the nucleus at the center. Notice that the 2s orbital is larger in diameter than the 1s orbital. In large atoms, the 1s orbital is nestled inside the 2s, just like the 2p is nestled inside the 3p. I can fully understand (a) in the above image of a QM model. moving on to (b) I get lost there is no 3p shown! would the 3p if shown only represent a smaller orbital? plus: the web page did not make me really understand the model. the only thing that the web page showed me was that the electron orbits in the same way as it always has. the 2p..3p ... and so on are only probability orbitals , not electron orbitals , and are only used to estimate where electrons might be as they orbit in 1s ...2s ....3s ... etc using mathematics. maybe that's why they use ( p ) for probability. if QM is just a means of estimation that can be utilize in actual equipment used to carry out experiments then it cannot claim that the bohr atom is incorrect as a atom model. the bohr atom model would be an incorrect model of a QM probability model however. using QM math to trigger a strobe light is probably how actual scientist see electrons as they orbit in s1..s2..s3 orbitals. but I think it is safe to say that actual electrons in orbit around an actual nucleus do not follow the orbital paths p , d shown in the QM atom model.
3/4 inch of dust build up on the moon in 4.527 billion years,LOL and QM is fantasy science.
|
|
|
|
|
Joined: May 2011
Posts: 2,819
Megastar
|

Megastar
Joined: May 2011
Posts: 2,819 |
You need to remember its 3D so they have mark x,y and z axis for (b) the electrons have 3 possible axis if they were all full they would form 3 clouds along each axis. There will be plenty of movies etc on youtube about it I am sure.
Electrons don't orbit in the planetary way you want to make them which is where you are running into problems. These are historic errors that come from you being taught errors. They spin in wave like patterns within those strange orbital shapes.
The problem dates back to 1922 and this experiment
http://en.wikipedia.org/wiki/Stern%E2%80%93Gerlach_experiment
The bohr model was dead the moment that experiment was done and no amount of explaining could save it the result is inconsistant with what would happen with a bohr type atom.
Since then we have experiment after experiment to isolate the quantum parameters and as I have said Bohr model is dead and buried.
There is also an obvious inconsistancy in Bohr model with antiparticles. How do you make an antipartilce molecule what the big protons orbit the small electrons in the middle :-)
So you have a choice now read about the various experiments that provide science proof of what is happening and try and understand it or do the Paul hand wave and stay with some outdated and clearly wrong idea.
So which is it to be try and understand or stick your head in the sand?
Last edited by Orac; 09/07/12 08:48 PM.
I believe in "Evil, Bad, Ungodly fantasy science and maths", so I am undoubtedly wrong to you.
|
|
|
|
|
Joined: Mar 2006
Posts: 4,136
Megastar
|

Megastar
Joined: Mar 2006
Posts: 4,136 |
Electrons don't orbit in the planetary way you want to make them which is where you are running into problems. These are historic errors that come from you being taught errors. They spin in wave like patterns within those strange orbital shapes. here is a actual image of an atom. it clearly shows that electrons orbit atoms the way planets orbit a sun.  doesnt look anything like one of the computer generated QM probability atom model's now does it. could it be that the model only focuses on the probability areas that the electron might be found in? a shadow of a Yb ytterbium atom  6 rings Yb ytterbium atom  as for hand waving I'm posting up mostly actual images of actual atoms other than a chart type image here and there as the above Yb atom chart. you are posting up computer generated images of imaginary atoms. I wouldn't call actual images of actual atoms hand waving or sticking my head in the sand. I would like to point out that your imaginary atoms must be somewhere in the sand , can you see them. do you see how your trolling extracted an emotional response from me above? this is why trolling is not allowed , granted I have been guilty of the same things in the past , I admit that , but I am trying to refrain from trolling , but your trolls are creeping in again , building momentum with your trolls. theres no need to include the type of language you use on a science forum. http://www.networlddirectory.com/blogs/p...-bohr-atom.html HOUSTON -- June 30, 2008 -- Nearly a century after Danish physicist Niels Bohr offered his planet-like model of the hydrogen atom, a Rice University-led team of physicists has created giant, millimeter-sized atoms that resemble it more closely than any other experimental realization yet achieved.
The research is available online in Physical Review Letters
Bohr offered the first successful theoretical model of the atom in 1913, suggesting that electrons traveled in orbits around the atom's nucleus like planets orbiting a star. Bohr's model led to a deeper understanding of both the chemical and optical properties of atoms and won him a Nobel Prize in 1922. But his notion of electrons traveling in discrete orbits was eventually displaced by quantum mechanics, which revealed that electrons don't have precise positions but are instead distributed in wave-like patterns.
"In a sufficiently large system, the quantum effects at the atomic scale can transition into the classical mechanics found in Bohr's model," said lead researcher Barry Dunning, Rice's Sam and Helen Worden Professor of Physics and Astronomy. "Using highly excited Rydberg atoms and a series of pulsed electric fields, we were able to manipulate the electron motion and create circular, planet-like states".
The team included members from Oak Ridge National Laboratory and Vienna University of Technology. Using lasers, the scientists excited potassium atoms to extremely high levels. Using a carefully tailored series of short electric pulses, the team was then able to coax the atoms into a precise configuration with one point-like, "localized" electron orbiting far from the nucleus. In fact, the atoms are true atomic giants, with diameters approaching one millimeter.
"Our measurements show that the electrons remain localized for several orbits and behave much as classical particles," Dunning said.
He said the work has potential applications in next-generation computers and in the study of classical and quantum chaos. I'm going to say that bohr was right all along and the sand bucket has been very busy for a long time. I can accept that the QM probability model of an atom can be used in determining where an electron might be in an atom. but I cant accept that the electrons could orbit an atom the way you seem to be trying to say that they do. it might be clear to QM but I just cant see that up here where the sand doesn't block my vision and perception. 
3/4 inch of dust build up on the moon in 4.527 billion years,LOL and QM is fantasy science.
|
|
|
|
|
Joined: Feb 2007
Posts: 1,840
Megastar
|

Megastar
Joined: Feb 2007
Posts: 1,840 |
There is also an obvious inconsistancy in Bohr model with antiparticles. How do you make an antipartilce molecule what the big protons orbit the small electrons in the middle :-) Despite a reluctance to back up the resident troll, I have to point out that even in the Bohr model an antiparticle molecule would consist of atoms with their constituent particles in the same relative locations as in normal atoms, with the same masses. Only the particles' charges would be be reversed. Positrons would be in orbit around anti-protons. Of course, in no way does that support the Bohr model, which as a matter of fact is now well and truly defunct, whether or not Paul likes it. But as we shall no doubt be reminded, Paul knows better than mere physicists.
"Time is what prevents everything from happening at once" - John Wheeler
|
|
|
|
|
Joined: Mar 2006
Posts: 4,136
Megastar
|

Megastar
Joined: Mar 2006
Posts: 4,136 |
Despite a reluctance to back up the resident troll In Internet slang, a troll is someone who posts inflammatory,[2] extraneous, or off-topic messages in an online community, such as a forum, chat room, or blog, with the primary intent of provoking readers into an emotional response[3] or of otherwise disrupting normal on-topic discussion.[4] The noun troll may refer to the provocative message itself, as in: "That was an excellent troll you posted." the remark you made about a resident troll is a troll! at least your post focuses on the scientific content and not the social aspects of the discussion. perhaps this forum might just survive as a science forum vs a social forum. or is that anti social forum!
3/4 inch of dust build up on the moon in 4.527 billion years,LOL and QM is fantasy science.
|
|
|
|
|
Joined: May 2011
Posts: 2,819
Megastar
|

Megastar
Joined: May 2011
Posts: 2,819 |
Despite a reluctance to back up the resident troll, I have to point out that even in the Bohr model an antiparticle molecule would consist of atoms with their constituent particles in the same relative locations as in normal atoms, with the same masses. Only the particles' charges would be be reversed. Positrons would be in orbit around anti-protons. Of course, in no way does that support the Bohr model, which as a matter of fact is now well and truly defunct, whether or not Paul likes it. But as we shall no doubt be reminded, Paul knows better than mere physicists.
Sorry as you can imagine this is all sort of confusing to me because it's not often I would get into discussion about how people percieve stuff that is wrong. It doesn't make alot of sense so what is "elementary charge" something that just occurs in the model like static electricity?
I believe in "Evil, Bad, Ungodly fantasy science and maths", so I am undoubtedly wrong to you.
|
|
|
|
|
Joined: May 2011
Posts: 2,819
Megastar
|

Megastar
Joined: May 2011
Posts: 2,819 |
but I cant accept that the electrons could orbit an atom the way you seem to be trying to say that they do.
Science says http://en.wikipedia.org/wiki/Stern%E2%80%93Gerlach_experiment YOU ARE WRONG .... BOHR MODEL HAS BEEN FALSIFIED. END OF STORY Not important what I and science believe the Bohr model is false and can not be upheld. If you can devise and experiment to falsify QM atomic model knock yourself out it's that simple and I would have to accept your result. The rules of science are that simple.
Last edited by Orac; 09/08/12 02:34 AM.
I believe in "Evil, Bad, Ungodly fantasy science and maths", so I am undoubtedly wrong to you.
|
|
|
|
|
Joined: Mar 2006
Posts: 4,136
Megastar
|

Megastar
Joined: Mar 2006
Posts: 4,136 |
If this value arises as a result of the particles rotating the way a planet rotates, then the individual particles would have to be spinning impossibly fast. Even if the electron radius were as large as 2.8 fm (the classical electron radius), its surface would have to be rotating at 2.3×1011 m/s. The speed of rotation at the surface would be in excess of the speed of light, 2.998×108 m/s, and is thus impossible.[2] LOL I suppose that's the body of your argument. well , if there were a atom on a space ship traveling at near the speed of light. say .9 c wouldnt the atoms electrons that travel at near the speed of light be traveling around the atom's nucleus faster than the speed of light durring 1/2 of each orbit or orbital in either or any model you choose? just suppose the electron were only traveling at .9 c around the nucleus. .9c + .9c = 1.8c !!! The rules of science are that simple. will the electron borrow some anti speed from the other side of the universe as it rounds the turn , just to prove that QM is correct even when its clearly wrong? LOL of course your talking about the spin of the electron and the above only talks about the electron as it orbits the nucleus. when we add in the speed of the surface of the electron that is traveling at 1.8c what would the surface speed be then. because the surface speed of 1/2 of each spin will probably be in the direction of travel at some point in time. then the individual particles would have to be spinning impossibly fast its funny how the impossible can become possible so quickly. I dont know about that , hide and watch! you guys are going to have to stop setting limits on things you dont have a clue about.
3/4 inch of dust build up on the moon in 4.527 billion years,LOL and QM is fantasy science.
|
|
|
|
|
Joined: Jun 2008
Posts: 415
Senior Member
|
OP

Senior Member
Joined: Jun 2008
Posts: 415 |
you guys are going to have to stop setting limits on things you dont have a clue about.
We are setting limits on things ( for example: c=1, T=0K ) that we don’t have a clue about. From these two parameters (c=1, T=0K ) was started all modern speculations in Physics. ==..
|
|
|
|
|









