17 October 2008
"Lost" experiment hints at a richer primordial stew
by Kate Melville
 A classic 50s experiment (known as the Miller-Urey Synthesis) that showed how amino acids could be created from inorganic molecules and an electrical spark isn't the whole story, it turns out. There were two related experiments, neither of which was reported at the time, which under modern analysis yield very interesting findings.
A classic 50s experiment (known as the Miller-Urey Synthesis) that showed how amino acids could be created from inorganic molecules and an electrical spark isn't the whole story, it turns out. There were two related experiments, neither of which was reported at the time, which under modern analysis yield very interesting findings.
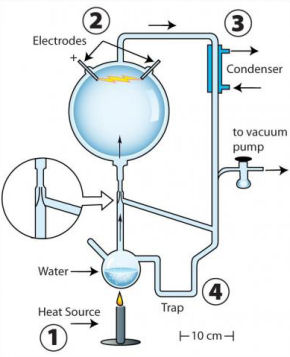
One of these initial experiments actually produced a wider variety of organic molecules than the experiment that made Stanley Miller famous. The difference between the two experiments is small - the unpublished experiment (pictured) used a tapering glass "aspirator" that simply increased air flow through a hollow, air-tight glass device. Increased air flow creates a more dynamic reaction vessel, or "vapor-rich volcanic" conditions, according to the present report's authors.
"The apparatus Stanley Miller paid the least attention to gave the most exciting results," said Adam Johnson, lead author of the new assessment that appears in Science. "We suspect part of the reason for this was that he did not have the analytical tools we have today, so he would have missed a lot." In his original 1953 article, Miller used a relatively primitive blotting technique to identify just five amino acids: aspartic acid, glycine, alpha-amino-butyric acid, and two versions of alanine.
Using modern analysis techniques, Johnson and his team identified 14 amino acids and five amines from the vials created from the original experiment. Even more impressively, however, the 11 vials from the unpublished experiment produced 22 amino acids and the same five amines. "We believed there was more to be learned from Miller's original experiment," co-researcher Jeffrey Bada said. "We found that in comparison to his design everyone is familiar with from textbooks, the volcanic apparatus produces a wider variety of compounds. Many of these other amino acids have hydroxyl groups attached to them, meaning they'd be more reactive and more likely to create totally new molecules, given enough time."
So what could be driving the second experiment's molecular diversity? And why didn't Miller publish the results of the second experiment? A possible answer to the first question may be the increased flow rate itself, Johnson explained. "Removing newly formed molecules from the spark by increasing flow rate seems crucial," he said. "It's possible the jet of steam pushes newly synthesized molecules out of the spark discharge before additional reactions turn them into something less interesting. Another thought is that simply having more water present in the reaction allows a wider variety of reactions to occur."
An answer to the second question is relegated to speculation - Miller, still a hero to many scientists, succumbed to a weak heart in 2007. Johnson says he and Bada suspect Miller wasn't impressed with the second experiment's results, instead opting to report the results of the simpler experiment.
Miller's third, also unpublished, experiment used an apparatus that had an aspirator but used a "silent" discharge. This third device appears to have produced a lower diversity of organic molecules.
Related:
Primordial Soup-In-A-Cup
Meteorites Spiced Up Primordial Soup
Between The Sheets
Volcanic Clay May Have Served As Womb For Emergent Life
Clay May Have Been Catalyst For Life
Source: Indiana University
