OK , I read the highly informative and scientific article
on the "NEW SCIENTIST" web site that you posted the link to.
we know that co2 is a greenhouse gas because it absorbs
and emits infrared radiation , basic physics tells us that
gasses with this property trap heat radiating from the earth...
basic physics tells us the following about the above MYTH !!!
lets reduce this process down to a earth that has only 1
co2 molecule in the atmosphere.
tell me at which time in the following basic physics processes
does the earth or the atmosphere warm due to the co2 molecule in the
atmosphere.
where t = time interval
and E = added energy
Earth = the earth
Atmos = the earths atmosphere
t=1
Earth E = 0
Atmos E = 0
sunlight passes by the co2 molecule on its trip to
the earths surface because its frequency is different
from the co2 molecule.
t=2
Earth E = 1
Atmos E = 0
the sunlight is absorbed by an object on the earth.
at this point for a fraction of a second there is a
tiny amount of heat transferred into the object on the
earth.
t=3
Earth E = .5
Atmos E = 0
a fraction of a second later the object then emits
infrared light (a photon of light)
at this point the object retains some of the heat energy
that it initially received from sunlight.
because the object released energy when it emitted the infrared light
t=4
Earth E = .5
Atmos E = .5
the infrared light that was emitted from the object
is then absorbed by the co2 molecule in the atmosphere.
at this point the co2 molecule becomes excited and undergoes
a frequency change ... and it cannot absorb another photon
until it emits a photon of the same energy that it absorbed.
for a tiny fraction of a second the co2 molecule moves around
in the atmosphere faster than it did before it became excited
by the infrared light that was emitted from the object on the earth.
t=5
Earth E = .5
Atmos E = 0
the co2 molecule then emits a photon with the exact energy
that it absorbed.
so far during this process the earth has gained .5 energy from the
visible light not the infrared light as the energy of the infrared light
is emitted by the object.
the co2 molecule has not gained any energy and the atmosphere has not
gained any energy.
so where in physics can it be claimed that co2 causes any warming?
the energy that is sent into the atmosphere (infrared) is not stored.
it is immediately released mostly back into space or towards the earth again.
now suppose the co2 molecule emits its infrared photon towards the earth
and the infrared photon is absorbed by the object on the earth.
t=6
Earth E = 1
Atmos E = 0
the infrared light is absorbed by the object on the earth.
and almost immediatly the object emits a infrared photon with the exact same energy.
t=7
Earth E = .5
Atmos E = 0
the photon of infrared light emitted from the object
is now traveling to the co2 molecule that is in the atmosphere.
--------------------------------------------------------------
this process can never change in a way that will cause
any additional energy (heat) to be stored on the earth or
in the atmosphere.
and it does not matter how many times the process is repeated.
and adding more co2 molecules to the atmosphere will deliver the same
results per each co2 molecule added.
note: where the belief , thoughts or claims that co2 causes any warming at all
is in question everything , all evidence , every claim and every word that exist above
the basic fundamental physical processes involved are muted debunked and totally
refuted by basic physics.
also:
if you desire to claim that the time interval between
t=4 and t=5 where the co2 molecule moves around in the
atmosphere faster and that an interaction with other
particles and/or molecules in the atmosphere causes some
degree of heat to build in the atmosphere then you will
need to explain where the energy that causes the heat comes
from ... because the co2 molecule must release a photon of
the same energy that it absorbed.
and as
we you all know
" you cant get a free lunch "
" you cant pull yourself up by your bootstraps "
and
" you cannot create energy "
etc ... etc ... etc ...
but your welcome to try if you like.





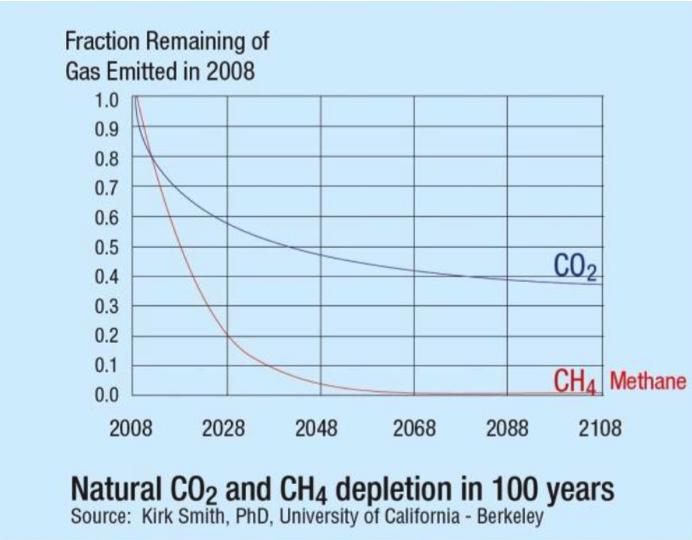
 ...since methane is measured in ppb (parts per
...since methane is measured in ppb (parts per 

