|
0 members (),
619
guests, and
1
robot. |
|
Key:
Admin,
Global Mod,
Mod
|
|
|
|
Joined: Mar 2009
Posts: 90
Member
|
OP

Member
Joined: Mar 2009
Posts: 90 |
An unusual battery is proposed in the experiment below. The particularity of this cell is the fact that both electrodes (formal cathode and anode) undergo oxidation phenomenon. The experiment is very simple: put a piece of Fe and a piece of Zn in a sulfuric acid solution and connect these pieces of metal to an ammeter. Although both metals are oxidized, an electric current is generated in an external circuit. The detailed description of the experiment is presented at www.elkadot.comA video is posted too, showing that bubbles of hydrogen develop at both metallic electrodes. It cannot be accepted that electrons are generated at both electrodes and these electrons are traveling in the external circuit in opposite directions only for the sake of traveling...meet each other, greet each other and continues on ... In the frame of actual science, no possible explanation can be formulated for the experiment and a new frame for conversion of chemical energy in electrical energy have to be proposed. You can perform the experiment in the kitchen using same metals and a acetic acid solution (vinegar). Regards,
|
|
|
|
|
Joined: Mar 2006
Posts: 4,136
Megastar
|

Megastar
Joined: Mar 2006
Posts: 4,136 |
move over Tesla , sorincosofret is moving in. Alternating Electricity !!! ever think of that? 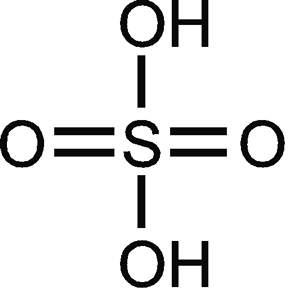 oh and the hydrogen that develops on the strips may be coming from the Sulfuric acid itself. the write up didnt say if the metal strips were being consumed or not , are they being consumed? if not then guess what you have here... $$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$ of course you must realize that its worthless in todays energy for profit world , if you could ask Nikola Tesla himself he would assure you of this fact. it would mean that you only buy 1 battery and you pass it down to your children when you die. --------------- also you didnt calculate the energy content in the released hydrogen which should move the 67 ma well beyond that number. and you could rig it up so that converting the hydrogen in a fuel cell would drop the resulting water back into the sulfuric acid solution , which would cause the solution strength to remain stable, and it would be a sealed battery that never needs refueling... --------------- it has no resale value. thats just the way the world is. it doesnt mean that you cant use it yourself or that you cant build it yourself and sell it , it only means that you wont be able to find funding for it. it also means that you might become paranoid about getting murdered and the paranoia itself might do you in. so just keep a low profile , apply for a patent , if its refused you at least have some proof of being the inventor. note: you may want to consider not applying for a patent and
just build and sell them to people who can keep a lid on it.
depending on which country you live in , and which country you live in might not even be a element to take into consideration these days.
and theres always the users of your ideas hidden away inside others ideas to increase energy efficiency , a personally known fact. but to have > 100% energy efficiency is a violation of idiot science. you must now decide if you want to be a idiot or not.
3/4 inch of dust build up on the moon in 4.527 billion years,LOL and QM is fantasy science.
|
|
|
|
|
Joined: May 2011
Posts: 2,819
Megastar
|

Megastar
Joined: May 2011
Posts: 2,819 |
Oh please it's a standard redox flow cell they have been known about since the 1970's. There is remotely nothing that can't be explained it is all completely known. You can also use vanadium, titanium, chromium and a number of other materials all of which have been investigated. http://electrochem.cwru.edu/encycl/art-b03-flow-batt.htmYou should also be aware there are many patents around the use of Zinc-Iron batteries in Europe, USA and Japan because these batteries can be scaled up for large grid backup 
Last edited by Orac; 10/20/13 06:07 PM.
I believe in "Evil, Bad, Ungodly fantasy science and maths", so I am undoubtedly wrong to you.
|
|
|
|
|
Joined: Mar 2006
Posts: 4,136
Megastar
|

Megastar
Joined: Mar 2006
Posts: 4,136 |
yep, it looks like it is alternating electricity!  thanks , orac. I didnt see the combination of materials that sorincosofret was using in the page you linked to. and he did say he was getting hydrogen at both electrodes. also he didnt say that his electrodes were being consumed. the only thing he needs is to figure out where the oxygen went and if need be feed oxygen into the mix and recombine the h and the oxygen to make a nice battery. after all these guys recieved funding so their ideas arent as powerful ! some may have even scaled back in order to get funding just like I was requested to do before.
3/4 inch of dust build up on the moon in 4.527 billion years,LOL and QM is fantasy science.
|
|
|
|
|
Joined: May 2011
Posts: 2,819
Megastar
|

Megastar
Joined: May 2011
Posts: 2,819 |
I didnt see the combination of materials that sorincosofret was using in the page you linked to.
and he did say he was getting hydrogen at both electrodes.
Equations for each electrode Zn + H2(SO4) ---> Zn(SO4) + H2 Fe + H2(SO4) -> Fe(SO4) + H2
also he didnt say that his electrodes were being consumed.
Actually he did he just used the scientific term "oxidized"
Although both metals are oxidized
What is driving the process self sustaining however is the redox between ZN and FE http://dl.clackamas.cc.or.us/ch105-09/calculat.htm
Next we have zinc metal reacting with iron(II) ion, which is just a little bit below the zinc on the list. In this case the zinc metal becomes zinc ion with a voltage of +0.76. Iron(II) ion becoming iron metal is the reverse of the reaction as written on the standard oxidation potential list. Thus, it will have a voltage of -0.44. The two half-reactions together then would have a voltage of those two added together (+0.76 and -0.44) giving a voltage of +0.32 volts.
These two examples are reactions which occur spontaneously. They are reactions with positive voltages. Redox reactions with negative voltages do not occur spontaneously. They must be forced.
So that is where the reaction is getting it's energy and why it occurs. Ultimately it will oxidize both electrodes away releasing there energy as it does.
after all these guys recieved funding so their ideas arent
as powerful !
some may have even scaled back in order to get funding just
like I was requested to do before.
I have no problem with them playing around but when they run across something they don't understand they could simply ask someone or an appropriate forum. What I disagree with is the whole we discovered something unbelievable hype which is almost always garbage. EDIT:I should add a comment for completeness the reactions above you can actually add a catalyst to the process if you mixed copper sulphate in the solution http://www.tutorvista.com/content/scienc...reactive-metalsYou will see both reactions listed there with a note
Zn(s) + H2SO4(g) -> ZnSO4(s) + H2(g)
(this reaction is catalysed by adding a trace of copper sulphate solution)
Slow reaction with dilute sulphuric acid forming the soluble pale green salt Iron (II) sulphate and hydrogen gas.
Fe(s) + 2H2SO4(g) -> FeSO4(s) + H2(g)
That should also give you an idea of what many of the patents are on 
Last edited by Orac; 10/23/13 06:29 AM.
I believe in "Evil, Bad, Ungodly fantasy science and maths", so I am undoubtedly wrong to you.
|
|
|
|
|
Joined: Mar 2009
Posts: 90
Member
|
OP

Member
Joined: Mar 2009
Posts: 90 |
Chemists are usually clear in their formulation ....
What is written as comments are not scientific comments.
Both electrodes are consumed, hidrogen develops at both electrodes...
So there it is a simple guess with no solution for actual chemistry:
Fe = Fe 2+ plus 2e which remains on the metalic electrode
Zn = Zn2+ plus 2e which remains on the metalic electrod.
According to actual chemistry electrons from Fe must travel at Zn electrode to generate hydrogen and electrons from Zn must travel to Fe electrode to generate hydrogen. How these electrons travel in opposite directions?
No answer...
So ... there remains the posibility that electrons from Fe react at the Fe electrode with hidrogen cation and form molecular hydrogen and electrons from Zn react at surface of Zn electrode and form molecular hydrogen ...
But in this case no charge is goind into extranal circuit so no electric current ...
Last edited by sorincosofret; 10/24/13 03:29 PM.
|
|
|
|
|
Joined: Mar 2009
Posts: 90
Member
|
OP

Member
Joined: Mar 2009
Posts: 90 |
I have read about these kind of batteries and my experiment is not the same ,,style”.
In thse batteries you have 2 metals one in in oxidised form an one in reduced form ... so there is a redox reaction ...
In my experiment both metals got oxidised .....and both metals start from a low state of oxidation.
Last edited by sorincosofret; 10/24/13 03:32 PM.
|
|
|
|
|
Joined: May 2011
Posts: 2,819
Megastar
|

Megastar
Joined: May 2011
Posts: 2,819 |
Let me be absolutely clear there are two processes happening As per http://www.tutorvista.com/content/scienc...reactive-metalsEither electrode will actually produce the hydrogen gas without the other electrode even in the solution TRY IT !!!! The reactions are actually completely independent of each other Zn(s) + H2SO4(g) -> ZnSO4(s) + H2(g) AND Fe(s) + 2H2SO4(g) -> FeSO4(s) + H2(g) What both of those reactions are doing is putting the metal in solution ZnSO4(s) AND FeSO4(s) So now you have Fe2+ and Zn2+ ions in solution and now you have a straight forward REDOX reaction creating the voltage Get it all you have done is combined the two effects in on container they are not the same process. You would actually improve the voltage and reactions by separating the solution into two jars joined by a salt bridge and dropping some copper sulphate in with the acid on zinc plate side. http://en.wikipedia.org/wiki/Salt_bridgeREDOX is quite often organized by multiple stage reactions there is nothing remotely surprising or mysterious about your experiment.
I believe in "Evil, Bad, Ungodly fantasy science and maths", so I am undoubtedly wrong to you.
|
|
|
|
|
Joined: May 2011
Posts: 2,819
Megastar
|

Megastar
Joined: May 2011
Posts: 2,819 |
For you sorincosofret here is your same trick done in school science experiments with fruit we usually use copper and zinc because it gives a higher voltage but iron and zinc will work http://msc-ks4technology.wikispaces.com/Fruit+Batteries  Wikipedia has a whole article about it http://en.wikipedia.org/wiki/Lemon_batteryIt even gives you possible substitutes
Zinc and copper electrodes are reasonably safe and easy to obtain. Other metals such as lead, iron, magnesium, etc., can be studied as well; they yield different voltages than the zinc/copper pair. In particular, magnesium/copper cells can generate voltages as large as 1.6 V in lemon cells. This voltage is larger than obtainable using zinc/copper cells. It is comparable to that of standard household batteries (1.5 V), which is useful in powering devices with a single cell instead of using cells in series.
So Zinc + almost any other metal will produce a voltage it will just differ slightly  So hopefully we are clear there are two process: So hopefully we are clear there are two process:
1: The oxidation of the electrodes producing the gas (a single electrode by itself) will do the same thing ... TRY IT! 2: The redox process creating the voltage And the memo ... no mystery to what is happening it is well understood at even school level!!!
And it has nothing to do with double oxidation at all.
Last edited by Orac; 10/25/13 02:16 AM.
I believe in "Evil, Bad, Ungodly fantasy science and maths", so I am undoubtedly wrong to you.
|
|
|
|
|
Joined: Mar 2006
Posts: 4,136
Megastar
|

Megastar
Joined: Mar 2006
Posts: 4,136 |
What both of those reactions are doing is putting the metal in solution ZnSO4(s) AND FeSO4(s) so you basically get electricity at no cost! and you get hydrogen ( more electricity ) at no cost. because the metals are not consumed. and its sustainable because nothing is consumed. so in order to get the metal out of solution what would you need to do? the hydrogen is coming from somewhere , Im going to guess its coming from the water in the sulfuric acid solution. I think he should continue to experiment , or he will wonder about it. http://www.webelements.com/zinc/chemistry.html Zinc metal dissolves slowly in dilute sulphuric acid to form solutions containing the aquated Zn(II) ion together with hydrogen gas, H2. In practice, the Zn(II) is present as the complex ion [Zn(OH2)6]2+. Zn(s) + H2SO4(aq) --> Zn2+(aq) + SO42-(aq) + H2(g) The reacts of zinc with oxidizing acids such as nitric acid, HNO3, are complex and depend upon precise conditions.
3/4 inch of dust build up on the moon in 4.527 billion years,LOL and QM is fantasy science.
|
|
|
|
|
Joined: May 2011
Posts: 2,819
Megastar
|

Megastar
Joined: May 2011
Posts: 2,819 |
The acid is becoming neutralized Paul  The whole thing will eventually stop because the acid will cease to exist. That is what is driving the process ... the hydrogen ions in the acid and that's why acids attack most substances. It's not a perpetual motion machine but you can drive the process backwards by putting the electricity in rather than extracting it. It really is a battery but for it when you run out of acid it's flat !!! The same reality exists for fruit batteries eventually the fruit runs out of citric acid  Sulfuric acid takes a lot of energy to make commercially mainly heating and catalyst manufacture. For our fruit battery the citric acid was made by a plant converting a hell of a lot of sunlight energy So you really aren't getting anything for free in any of these processes they all required a huge energy input to get the pittance of energy you are drawing 
Last edited by Orac; 10/25/13 09:23 AM.
I believe in "Evil, Bad, Ungodly fantasy science and maths", so I am undoubtedly wrong to you.
|
|
|
|
|
Joined: Mar 2006
Posts: 4,136
Megastar
|

Megastar
Joined: Mar 2006
Posts: 4,136 |
It really is a battery, but when you run out of acid it's flat !!! but you can drive the process backwards by putting the electricity in rather than extracting it. and all the while there is hydrogen being produced by the process. are you attempting to say that there is more electricity needed to drive the process backwards in order to replenish the metal electrodes? needless to say there will be pressure and/or vacuum involved in the mix as well along with temperature. and just how is the acid removed? wouldnt the acid also be replenished by driving the process backwards? or does the acid just vanish into thin air? by utilizing the hydrogen produced at the electrodes and a fuel cell that converts the hydrogen and oxygen into H2O and the H2O drops back into the sulfuric acid solution how would the acid solution become dilute? how would you ever run out of acid? and the battery should produce electricity as long as there is still a coating of zinc and fe on their respective electrodes. it looks like a pretty good battery to me. it should be rechargeable and never needs maintenance. it should never wear out either !!! thus...no funding( no resale value). nothing ever actually leaves the battery. the only thing that is occurring outside the sealed battery is the movement of electrons and heat transfer which can be greatly reduced with proper design and insulation.
3/4 inch of dust build up on the moon in 4.527 billion years,LOL and QM is fantasy science.
|
|
|
|
|
Joined: May 2011
Posts: 2,819
Megastar
|

Megastar
Joined: May 2011
Posts: 2,819 |
I do find your understanding of what is happening and the world around you quite amusing  Do you really want me to explain it or are you happy in your own little LALA world. Recharging ANY rechargeable battery involves more energy than it consumes because there are losses and this battery is not immune from the problem. So lets set you a challenge see if you can work out areas of energy loss or are you stupid enough to think you can actually build a perpetual motion machine  Edit: Edit: A friendly material scientist gave me the typical loss for any battery charging which is 20-25%. That number is consistent across Li/Ion, Lead acid and NiCad batteries. I will give you hints to a couple of the problems which stem around the error in this statement
nothing ever actually leaves the battery.
the only thing that is occurring outside the sealed battery
is the movement of electrons and heat transfer which can be
greatly reduced with proper design and insulation.
1.) RADIOACTIVE DECAY http://en.wikipedia.org/wiki/Isotopes_of_zinc
Naturally occurring zinc (Zn) is composed of the 5 stable isotopes 64Zn, 66Zn, 67Zn, 68Zn, and 70Zn with 64Zn being the most abundant (48.6% natural abundance). Twenty-five radioisotopes have been characterised with the most abundant and stable being 65Zn with a half-life of 244.26 days, and 72Zn with a half-life of 46.5 hours. All of the remaining radioactive isotopes have half-lives that are less than 14 hours and the majority of these have half-lives that are less than 1 second. This element also has 10 meta states.
 2. CORROSION http://en.wikipedia.org/wiki/Rust
Given sufficient time, oxygen, and water, any iron mass will eventually convert entirely to rust and disintegrate.
 So now lets deal with the acid problem for you: So now lets deal with the acid problem for you:1.) Hydrogen leakage. The hydrogen molecule is so small it leaks thru any container slowly it is simply a matter of time. Being an acid the hydrogen molecule is only loosely bonded and is in solution as a free radical often and it leaks very badly. The process adds to the next problem 2.) Reaction of hydrogen ions with contaminations. The hydrogen ions will eventually find any impurities in the container, metal plates and solution. If you want a sort of basic background the science of hydrogen and trying to stop it's damage has it's own page http://en.wikipedia.org/wiki/Hydrogen_damageSo got a hydrogen proof container and metal plates?  THERE ARE A LOT MORE BUT THATS A START THERE ARE A LOT MORE BUT THATS A START
Most rechargeable batteries are rated on how many recharges they will survive and an absolute time http://en.wikipedia.org/wiki/Rechargeable_batteryOur batteries above will have the same limitations Edit: Our friendly material scientist gave me a rough guess on an iron/zinc battery compared to a lead acid at about 1/3rd the life expectancy for similar grade materials and construction. The increased failure expectancy due to the increased reactivity of the zinc plate with all sorts of things and increased sulfating of the plate. The world isn't perfect everything DIES and you never get back the energy you put into any system.. There is only one anecdotal recording of anyone ever getting out more energy than they put in .... this bloke did a thing on the banks of a river with bread and fish but I heard it was a hoax 
Last edited by Orac; 10/25/13 11:05 PM.
I believe in "Evil, Bad, Ungodly fantasy science and maths", so I am undoubtedly wrong to you.
|
|
|
|
|
Joined: Mar 2006
Posts: 4,136
Megastar
|

Megastar
Joined: Mar 2006
Posts: 4,136 |
I think your taking this to the extreme orac when you are
suggesting that radioactive decay would be a real problem.
its almost as if you are trying to say that if you purchase
a supply of zinc by the time you get the shipment it has
already disappeared because of radioactive decay.
how many millions of years would actually pass before a
significant amount of zinc would actually decay away?
and plus ... I only said that it would be perpetual
IF THE ELECTRODES WERE NOT BEING CONSUMED!!!!!!!!!!!!!!!!!!
but we later found that they were being put into solution by the reaction.
thus recharging would be required.
(however I found a way around the need for recharging)
now for your "the acid problem"
hydrogen leakage , LOL , Im beginning to realize that you may
be interested in this idea , you have even introduced a
Our Friendly Material Scientist Guy to blow smoke up the arses of readers.
the facts are that the materials will not just disappear inside the battery , no matter how much you want them to.
the materials are simply transformed.
and yes , Im happy in my LALA world as you would naturally call it , because it has rules that dictate the way things work and those rules are not simply created by the type
of scientist that exist in the world today when ever they
need a situation to play out a certain way.
3/4 inch of dust build up on the moon in 4.527 billion years,LOL and QM is fantasy science.
|
|
|
|
|
Joined: May 2011
Posts: 2,819
Megastar
|

Megastar
Joined: May 2011
Posts: 2,819 |
Whatever Paul.
It's like discussing any mechanical problem and ignoring friction and other losses it's fine to do that for a general discussion if that's what you are discussing.
However if you suggest that you can build a mechanical perpetual motion machine the answer is no you can't and these are the reasons why. Your answer seemed to suggest perpetual motion and a battery that was somehow better than existing ones and specifically it isn't based on life expectancy and why you don't see this battery on the market except in a very specific role for which it has advantages.
So my discussion may be over the top or not depending on which level you are doing, we often discuss mechanical things ignoring friction and the like in exactly the same way.
The chemical losses are like the mechanical losses real and measurable and I have given you and indication of the value around 20% of the energy exchanged via a friendly materials scientist.
The battery will also degrade for many and various reasons. Lead acid batteries are quoted as 5-8 year life cycle and zinc iron would be expected to be less that's what the materials people say.
Whether that is important or not or you choose to believe it I leave up to you I simply provided the information as is as accurately as I can.
Last edited by Orac; 10/27/13 02:13 AM.
I believe in "Evil, Bad, Ungodly fantasy science and maths", so I am undoubtedly wrong to you.
|
|
|
|
|
Joined: Mar 2009
Posts: 90
Member
|
OP

Member
Joined: Mar 2009
Posts: 90 |
I have asked to have the possibility to clean up this discusson.
If someone feels comfident to develot the perpetual motion subject or etc etc .. please post another topic...
|
|
|
|
|
Joined: Mar 2006
Posts: 4,136
Megastar
|

Megastar
Joined: Mar 2006
Posts: 4,136 |
It cannot be accepted that electrons are generated at both electrodes and these electrons are traveling in the external circuit in opposite directions only for the sake of traveling...meet each other, greet each other and continues on ... I have ordered the materials to do the experiment myself. I will of course change a few things here and there to make a more efficient energy storage battery system. and since you want to clean up the discussion because you are bound to common knowledge pertaining to the energy industry , I will leave the discussion to you and orac so the two of you can discuss the impossibilities of achievement. have fun BTW , Orac if zinc and Iron are not suitable for a redox battery according to your friendly materials science guy then why do the battery systems in the image you posted use Zinc and Iron? LOL  it does say zinc iron now doesnt it? Im going to take a wild guess and say or claim that hydrogen is being produced inside these units , but in order to comply with common energy industry knowledge the hydrogen is being vented off rather than being used as a energy source. these days a lead acid car battery has a fuel cell inside it already to convert the oxygen and hydrogen produced inside the battery back into water its called a Catalytic gas recombiner made commercially available in the 1970s. In the case of lead acid cells, the term "valve-regulated cells" is more accurate, because they cannot be sealed completely. If they were, the hydrogen gas would cause the pressure to build up beyond safe limits. Catalytic gas recombiners do a great deal to alleviate this problem. They convert the hydrogen and oxygen back into water, achieving about 85% efficiency at best. Although this doesn't entirely eliminate the hydrogen and oxygen gas, the water lost becomes so insignificant that no refill is needed for the life of the battery. For this reason , these cells are often referred to as maintenance-free batteries. Also, this cell design prevents corrosive fumes from escaping.
3/4 inch of dust build up on the moon in 4.527 billion years,LOL and QM is fantasy science.
|
|
|
|
|
Joined: May 2011
Posts: 2,819
Megastar
|

Megastar
Joined: May 2011
Posts: 2,819 |
I will answer only one of your question as this is sorincosfret's thread and will follow his guidelines.
The reason Zinc-iron is used for online storage is the size of the capacity. Iron being credibly strong and easy to get makes the plates at that size and capacity a much better proposition than lead.
That is the commercial reason for the batteries to be used in this situation.
I will leave the rest alone.
I believe in "Evil, Bad, Ungodly fantasy science and maths", so I am undoubtedly wrong to you.
|
|
|
|
|
Joined: Mar 2006
Posts: 4,136
Megastar
|

Megastar
Joined: Mar 2006
Posts: 4,136 |
No layman has ever changed science, get a degree before you try and fix or rewrite science please. Nikola Tesla , did not get a college degree. Thomas Alva Edison, did not get a college degree. the above 2 are the most accredited laymen in history when accomplishments in science are concerned. they did not get , nor did they need a college degree and from what I have observed if they would have had a degree they may have never accomplished the things that their layman minds accomplished. Im not saying that people should not get a degree , Im just saying that changing science does not require a college degree it only requires the ability to think. something that college graduates cant get by getting a college degree. besides most inventions that change science or ideas that change science are stolen or borrowed from laymen who have the ability to think anyway. so if a layman desires to change science all he needs to do is to inform scientist about his ideas , greed and self satisfaction does the rest. it never fails. the layman has then changed science through a specific set of proxies. he has still changed science. back to my LALA world , proxy
3/4 inch of dust build up on the moon in 4.527 billion years,LOL and QM is fantasy science.
|
|
|
|
|
Joined: May 2011
Posts: 2,819
Megastar
|

Megastar
Joined: May 2011
Posts: 2,819 |
None of this has anything to do with the thread so I will make this brief and suggest perhaps you should read up on Edison and Tesla. Tesla attended university he did not get a degree because he failed the last semester due to his gambling addiction. http://en.wikipedia.org/wiki/Nikola_Tesla=>Tesla was unprepared and asked for an extension to study, but was denied. He never graduated from the university and did not receive grades for the last semester. Edison because of hearing problems never got to university but he was very bright and surrounded himself with intelligent people including Nikola Tesla. He was given an honary degree and admitted to the Royal Swedish Academy of Sciences in 1890. You may check the facts. The world being a lot different place back then and schooling rare I will cut them a little slack but they were both very familiar with science process something you sadly lack. Now out of respect to the thread owner lets stop there if you wish to discuss it further create a thread and I will happily argue with you.
I believe in "Evil, Bad, Ungodly fantasy science and maths", so I am undoubtedly wrong to you.
|
|
|
|
|







