|
0 members (),
632
guests, and
1
robot. |
|
Key:
Admin,
Global Mod,
Mod
|
|
|
|
Joined: May 2010
Posts: 410
Senior Member
|

Senior Member
Joined: May 2010
Posts: 410 |
My problems is probably the same as most people no organism we see around us today has survived the 4.5 billion years their genetic sequences arguably has but not them unchanged. However, they are all descended from ancestor which first arose ~4 billion years ago. Take any living organism alive today - whether it is a bacterium, or a human, or something else, they are all linked by an unbroken chain of living organisms back to the last common ancestor. Yes, we are different then our ancestors, but at no point is there a stage of non-life between any extant organism and the first organisms on earth. So calling any organism "higher" or "lower" is naive at best - we've all run the gauntlet of evolution for the same length of time, and all been successful at it. The only difference between "higher" and "lower" is the number of cells we happened to end up with. The oldest organism I know of unchanged is a stromatolite but I guess there may be some simple cell stuff that really does go across the 4.5 Billion years and so really has been successful as you define it. Actually, the bacterium which form stromatolites evolve rapidly, as do most bacteria. They have stumbled on a good survival strategy, so that aspect of their biology hasn't changed - but the remainder of their biology has evolved quite significantly. Much of what we know of cyanobacterium evolution has been discovered by studying the cyanobacterium in stromatolites. You can actually track their evolution by comparing sequences from different depths within the stromalite. Certain genetic sequences and approaches may be successful if we are defining existing for 4.5 Billion years but you can't really extend that to the organism. You're making a fundamental mistake here - assuming the genomic elements are more stable than species. The opposite is true - genomes change far faster than species. Life is not a static thing - it changes with every generation. Ergo, one should expect change over time - stasis = extinction, change = a chance to survive. The plasciticty that evolved early in lifes development is the very key to its success. Secondly you equate bacteria and more complex multi-cellular organism as the same based on one criteria that we are here today so we are all successful. In itself nothing wrong with that from a survival point of view but it overlooks the battle the more complex organisms underwent to be here. The more complex an organism the more thing there are to be attacked so the more advanced must be the mechanisms to protect it. It is therefore natural to view complex organism as more advanced because they face tougher risk/reward equations. Sorry, but that is completely backwards. Selection forces on bacterium are far stronger than that observed in multicellular organisms - indeed, the rates of genetic change, speciation & extinction are orders of magnitude higher in single celled organisms; a clear sign of stronger evolutionary impacts measured over the same period of time. To put it into context, your average bacterial species lasts from tens of thousands to a few hundreds of thousands of years; your average metazoan (animal) species lasts from 2-3 million (mammals) to 16-25 million years (formernera). I guess the way to pose the question back to you both is why do complex organisms exist at all, what is the payoff for being a complex organism and why did it come about?
The first thing to keep in mind is the vast majority of organisms - whether measured as total number of individuals, total number of species, or total biomass - are single-celled organisms (on a scale of 100:1 to trillions:1, depending on your choice of measurement). Meaning that multicellularity, as a survival mechanism, is not overly successful. The question isn't so much why it came about, but rather why it is such a rare evolutionary path. In terms of why it arose, predation is thought to be the primary driver - unicellular predators like amoebas cannot eat things larger than a few cells in size, so a simple multicellular organism like a sponge, or a pseudo-multicellular organism like choanoflagellates, can avoid a strong selective force simply by "clumping". After that, in-clade competition (i.e. competition between multicellular organisms) appears to have driven much of the rest of the evolutionary processes. Indeed, if you look at most of what has happened since we became multicellular, most of the adaptations have been to compete against other multicellulars. Bryan
UAA...CAUGCUAUGAUGGAACGAACAAUUAUGGAA
|
|
|
|
|
Joined: May 2011
Posts: 2,819
Megastar
|

Megastar
Joined: May 2011
Posts: 2,819 |
So calling any organism "higher" or "lower" is naive at best - we've all run the gauntlet of evolution for the same length of time, and all been successful at it. The only difference between "higher" and "lower" is the number of cells we happened to end up with.
Again you are missing my argument .. it will be clarified when I discuss you answer to my last question.
The plasciticty that evolved early in lifes development is the very key to its success.
Again I will pick this up because I agree it's important.
Sorry, but that is completely backwards. Selection forces on bacterium are far stronger than that observed in multicellular organisms - indeed, the rates of genetic change, speciation & extinction are orders of magnitude higher in single celled organisms; a clear sign of stronger evolutionary impacts measured over the same period of time. To put it into context, your average bacterial species lasts from tens of thousands to a few hundreds of thousands of years; your average metazoan (animal) species lasts from 2-3 million (mammals) to 16-25 million years (formernera).
I don't buy that they are stronger on the bacterium at all I believe they have less mechanisms with which to adjust to enviroment changes or competition ... hence they die out as a species faster. If you go forward to humans we can even control our enviroment to a high degree. This was what I was getting at a complex organism has more things to attack and normal science entropy logic says it should be weaker BUT IT ISN'T that means something is important going on here .. so you missed my point. You need to look at how a complex thing can be more robust than a simple thing because in the real world they never are unless they are engineered ... and no I don't believe in inteligent design 
The first thing to keep in mind is the vast majority of organisms - whether measured as total number of individuals, total number of species, or total biomass - are single-celled organisms (on a scale of 100:1 to trillions:1, depending on your choice of measurement). Meaning that multicellularity, as a survival mechanism, is not overly successful. The question isn't so much why it came about, but rather why it is such a rare evolutionary path.
I would have thought that it is obvious why it is rare go back to all your own comments above.
In terms of why it arose, predation is thought to be the primary driver - unicellular predators like amoebas cannot eat things larger than a few cells in size, so a simple multicellular organism like a sponge, or a pseudo-multicellular organism like choanoflagellates, can avoid a strong selective force simply by "clumping". After that, in-clade competition (i.e. competition between multicellular organisms) appears to have driven much of the rest of the evolutionary processes. Indeed, if you look at most of what has happened since we became multicellular, most of the adaptations have been to compete against other multicellulars.
And I totally agree with the above which when you put your own answers together it tells you why the complex organisms are considered advanced or higher. Using your own logic statements my argument goes like this - The simple organisms having only simple attack and defensive abilities could not out compete some complex organisms which developed and forced out a survival niche. - These early simple organisms had plasticity that was the key to there success but it also put some limits on enviroment which more complex organisms could adapt to with more complex structures, behaviours etc. - Ultimately more complex organisms tend to survive as a species longer because they have more ways to solve the survival issues. All of that defines complex organisms as being more advanced because they forced there way into survival against all the odds and now survive longer as a species than simple organisms. If you take this back to simple cars a Model T ford and the latest ferrari are equally successful they both exist today but trying to deny the Ferrari is not more advanced than the Model T is denying the obvious.
Last edited by Orac; 02/26/13 04:31 PM.
I believe in "Evil, Bad, Ungodly fantasy science and maths", so I am undoubtedly wrong to you.
|
|
|
|
|
Joined: May 2010
Posts: 410
Senior Member
|

Senior Member
Joined: May 2010
Posts: 410 |
I don't buy that they are stronger on the bacterium at all I believe they have less mechanisms with which to adjust to enviroment changes or competition ... hence they die out as a species faster. If you go forward to humans we can even control our enviroment to a high degree. There is no other answer to this other than "you are wrong". The degree of selection we see is a direct measure of the degree of selective forces experienced by the organism. "Compensatory mechanisms" like the ones you claim we use to somehow be stronger than bacteria are evolved - and often selected - traits and thus show the same evolutionary fingerprints as those observed in bacteria. One is not separate from the other. As a "rule" (because there are exceptions), bacteria are much more hardy than us, and can survive much wider swings in environmental conditions. And yes, they have compensatory mechanisms just like ours that allow them to do that. Take the lowly e coli - it can survive temperatures from well below freezing to ~40C - a far wider range than most multicellular organisms including ourselves. It can survive transient exposure to pH's as low as 1 and as high as 10, and can thrive in pH 4-8.5; most metazoans are stuck with 7 to 8; above/below that and we die. Same it true of salt concentrations, exposure to heavy metals & other environmental toxins, radiation exposure, etc, etc, etc. And e coli is average as bacteria go. Some survive & thrive over much broader temperature, pH, radiation, etc ranges. By any measure - whether its directly quantifying selection's impact on the genome, or by measuring resiliency to environmental stressors/competition/etc, bacteria generally show a degree of fitness far beyond that of metazoans. This was what I was getting at a complex organism has more things to attack and normal science entropy logic says it should be weaker BUT IT ISN'T that means something is important going on here .. so you missed my point. No, I didn't miss your point. Nor did I say that 'nothing important' occurred in the development of multicellularity. However, you did just display a further misunderstanding of what science actually says about evolution, and you continue to over-estimate the relative importance - and difficulty - of multicellularity. Entropy says nothing about the susceptibility of systems to interruption; all it describes is energy flow in a system. So there is no expectation that 'complex' organisms should be more or less susceptible to selective forces (which I assume is what you mean by 'weaker'). Secondly, the number of 'things' you have to 'attack' has no bearing on your evolutionary fitness - for several reasons: Firstly, more does not always mean more targets for attacks(and thus, greater susceptibility) - or vice-versa. In some cases additional traits allow compensatory responses, thereby decreasing your susceptibility relative to a less 'complex' organism. But more complexity can equal more potential targets for selection. 'Complexity' is a double-edged sword, rather than a universal panacea. Secondly, the number of "things" (genes) an organism has has no bearing on its susceptibility to selective forces. Selective forces are largely beyond the control of the organism experiencing it. You need to look at how a complex thing can be more robust than a simple thing because in the real world they never are unless they are engineered ... and no I don't believe in inteligent design  Firstly, you cannot equate human-designed objects to life - there is no semblance and the same rules do not apply. Secondly, you are falsely assuming that we have more 'parts' to break than simple organisms, and thus are more susceptible. The 'parts' evolution acts upon are genes - of which the "lowly" choanoflagellate has ~10,000 genes, and the "lowly amoeba has ~50,000. We have somewhere between 18,000 to 23,000 genes - less than some of those organisms you dismiss as 'simple'. Thirdly, you assume that 'complexity' solely leads to suceptablity. This is false - indeed, increased complexity often provides additional 'options' for dealing with stressors - i.e. given the same selective force, the change in fitness (and thus th degree of selection experience) is less in a 'complex' organism than in a 'simple' one.
The first thing to keep in mind is the vast majority of organisms - whether measured as total number of individuals, total number of species, or total biomass - are single-celled organisms (on a scale of 100:1 to trillions:1, depending on your choice of measurement). Meaning that multicellularity, as a survival mechanism, is not overly successful. The question isn't so much why it came about, but rather why it is such a rare evolutionary path.
I would have thought that it is obvious why it is rare go back to all your own comments above. Its rare because it requires very specific conditions to occur, and those conditions must occur over a long enough period for the multicellularity to become obligate - i.e. the conditions amiable to its formation are rare. Even so, those conditions have poccured - independently - at least three time in lifes history (plants, animals, fungi). Its rarity has nothing to do with its suceptability to selection - otherwise, there would be no - as in zero - multicellular species. And I totally agree with the above which when you put your own answers together it tells you why the complex organisms are considered advanced or higher. No it doesn't. Hubris tells us why they are considered higher or advanced. A basic understanding of evolution tells us those terms are fundamentally flawed.
Using your own logic statements my argument goes like this
- The simple organisms having only simple attack and defensive abilities could not out compete some complex organisms which developed and forced out a survival niche.
The attack and defensive abilities of most unicellular organisms are as complex and deadly as those we have, so your first assumption is false. - These early simple organisms had plasticity that was the key to there success but it also put some limits on enviroment which more complex organisms could adapt to with more complex structures, behaviours etc. Quite the opposite - the formation of multicellular organisms was contingent on the environment created by the unicellular ones.
- Ultimately more complex organisms tend to survive as a species longer because they have more ways to solve the survival issues.
Nope. Multicellular organisms evolved to deal with a single selective force; it was one route of many to deal with predation. All of that defines complex organisms as being more advanced because they forced there way into survival against all the odds and now survive longer as a species than simple organisms. Again, wrong. All extant organisms were forced to survive against the same odds; the only difference is the strategy used. That is the point you continually miss - anything alive today had ancestors which survived the same sets of environmental conditions. The e coli in your gut and you have run the evolutionary gamut, in the same environment, for the same amount of time. And you've also made a fatal error/fell into a trap. Measuring the success of an organism in years is meaningless, as years are not the 'scale' by which evolution measures time. In the 'units' of evolutionary time, unicellular species tend to last longer... ...lets see if you can figure out what those units of time are...
If you take this back to simple cars a Model T ford and the latest ferrari are equally successful they both exist today but trying to deny the Ferrari is not more advanced than the Model T is denying the obvious.
There are a number of flaws in your argument. Firstly, cars do not evolve, so the comparison is mute. Secondly, evolutionarily speaking the model t is extinct - no longer in production (reproducing) and thus dead as a species. Of course an extant species still thriving would be considered more advanced/better than an extinct one. And thirdly, your argument for 'advanced' is purely subjective - one could argue the ferrari is less 'advanced' due to its lesser utility, or shorter apparent species "lifespan" than the model 'T'. The later is a perfect example of why those of us in the biosciences avoid terms like 'advanced', 'higher', etc - whether something is 'advanced' or 'higher' is purely subjective, and depends on the criteria by which you base your measurements. By measure of top speed, humans are more advanced than yeast. As measured by alcohol tolerance, yeast are far more advanced than humans. Bryan
UAA...CAUGCUAUGAUGGAACGAACAAUUAUGGAA
|
|
|
|
|
Joined: May 2011
Posts: 2,819
Megastar
|

Megastar
Joined: May 2011
Posts: 2,819 |
Even if I accepted all your arguments which I don't you would still be wrong because of this point.
There are a number of flaws in your argument. Firstly, cars do not evolve, so the comparison is mute. Secondly, evolutionarily speaking the model t is extinct - no longer in production (reproducing) and thus dead as a species. Of course an extant species still thriving would be considered more advanced/better than an extinct one. And thirdly, your argument for 'advanced' is purely subjective - one could argue the ferrari is less 'advanced' due to its lesser utility, or shorter apparent species "lifespan" than the model 'T'.
Products do evolve it's even studied in most business schools. It is not the exact same thing obviously because you are correct they don't reproduce but they are subject to a selection process by consumers and they are tested by fitness for job. Extinction means they don't exist the Model T still exists in many museums and car clubs. Yes it's not in production so it would be in and critically endangered species in your talk because one can never tell maybe the world tomorrow may fall in love with it and it may be back in production then. This is all really word play but that is what alot of your argument to me is. No person or scientist I know would ever class a Model T more advanced than a modern Ferrari and that is why your argument becomes word play no sensical. The fact you consider by some word play you can invert and invent a criteria you can reverse that tells you that you are contriving an answer that noone would readily accept. Thus arguing this becomes pointless because most of it is nothing more than word play. I will have one last comment at the most wrong thing you have ever written from a science point
Entropy says nothing about the susceptibility of systems to interruption; all it describes is energy flow in a system. So there is no expectation that 'complex' organisms should be more or less susceptible to selective forces (which I assume is what you mean by 'weaker'). Secondly, the number of 'things' you have to 'attack' has no bearing on your evolutionary fitness - for several reasons:
Firstly, more does not always mean more targets for attacks(and thus, greater susceptibility) - or vice-versa. In some cases additional traits allow compensatory responses, thereby decreasing your susceptibility relative to a less 'complex' organism. But more complexity can equal more potential targets for selection. 'Complexity' is a double-edged sword, rather than a universal panacea.
Secondly, the number of "things" (genes) an organism has has no bearing on its susceptibility to selective forces. Selective forces are largely beyond the control of the organism experiencing it.
If you believe all of that you need to go and do a huge refresher coarse on Entropy and science it's meaning and what it implies because you are as you would say are "simply wrong". Your logic defies Entropy and in my area Quantum Mechanics your idea is in direct conflict with all it's central tenants. I know QM does not stop because something is alive and as QM has been much more rigorously tested than evolution I can say quite certainly you are wrong. QM states a system in a highly complex state is probably far from equilibrium and in a low entropy (improbable) state, where the equilibrium state would be simpler, less complex, and higher entropy. You state multicellular is rare and the above explains why if you hadn't realised it before that is the reason. Hydrogen is the most abundant molecule in the universe because it is the most simple stable state same exact reason. Ethan Segeil did a really good article on it which you should read http://scienceblogs.com/startswithabang/2013/01/02/what-we-learn-just-by-being-here/We are here because of the most unlikely of unlikely events being the triple-alpha process ( http://en.wikipedia.org/wiki/Triple-alpha_process). The fallout of the stability decay of all chemicals due to entropic principles means that every atom or molecule in the human body is replaced every 7 to 10 years that is a huge cost to the organism and it is not optional because thats the decay constant of the molecules in the universe the more complex they are the shorter they survive. ( http://en.wikipedia.org/wiki/Radioactive_decay)
A disturbance would thus facilitate the path to a state of greater entropy: The system will move towards the ground state, producing heat, and the total energy will be distributable over a larger number of quantum states
If you want some scale of the problem in humans we are made up of 9.5% carbon which is around around 10^26 to 10^27 atoms ( http://en.wikipedia.org/wiki/Composition_of_the_human_body). The half life of carbon-14 is 1.808*10^11 seconds which equates to around 5000 decays per second that your body must replace. Potassium comes in next at around 4500 decays per second this is actually a routine lab test ( http://sciencedemonstrations.fas.harvard...gecontent270775). Your body is committed to these replacements every second of every day it is alive awake or asleep the entropic principles involved are simply massive. As an aside when people talk about Silicon based life forms these decay rates are the reasons against it. The half life of Carbon-14 is 5700 years the half life of Silicon-32 is a mere 170 years. So if humans were silicon based life forms the entropic overhead is orders of magnitude higher again than a carbon based life form just based on the increased turn over for the organism to stay alive. This force is far far beyond the pathetic level of your selective forces it occurs in very short timeframes certainly less than the lifespan of most organisms and life is not exempt from the law. Thus a complex organism is facing a mountain compared to a simple organism just based on what has to be replicated on an ongoing basis by neccessity just to stay alive. Ultimately everything dies because it loses the battle to this law and even the universe itself is not excluded from it. If your selective forces were stronger than QM radioactive decay laws you would have species extinctions on average shorter than a few years and by your own comments its thousands of years. If perhaps your interested in why everything decays probably start here with Matt Strassler http://profmattstrassler.com/articles-an...-yet-some-dont/Your whole argument thus falls to the science axe of Occam's razor.
Last edited by Orac; 02/27/13 05:23 AM.
I believe in "Evil, Bad, Ungodly fantasy science and maths", so I am undoubtedly wrong to you.
|
|
|
|
|
Joined: Aug 2010
Posts: 3,570
Megastar
|

Megastar
Joined: Aug 2010
Posts: 3,570 |
I have just returned from 10 days in hospital with pneumonia. Eagerly I looked at SAGG. Imagine my disappointment when, reading through a range of recent posts, I found the old familiar round of political flatulence, petty, blinkered jingoism, ad hominem sniping and self-preening verbosity. I found myself wondering if it was worth looking for any science, or resuming posting in SAGG. Then I began reading this thread. Definitely not my subject, I have not dabbled in genetics since S101 (Open University, 1983), apart from a little that attached itself to the fossil record. It is refreshing to read a lively discussion which includes at least one person with professional knowledge. So much to learn! I was surprised to discover that I had posted in this thread. That probably reflects my state of health at the time. Unless having a sore back has a fitness cost (i.e. leads to fewer offspring) Any male who had serious back problems in his more athletic reproductive years would have some bitter comments on that subject.  ..lets see if you can figure out what those units of time are... Generations? Perhaps not as off topic as it might seem; while in hosp I read Adam Frank's "About Time". I recommend it highly. An easy read, different approach and lots of stuff to think about; including thoughts on why we humans evolved as we did.
There never was nothing.
|
|
|
|
|
Joined: May 2011
Posts: 2,819
Megastar
|

Megastar
Joined: May 2011
Posts: 2,819 |
Ohh sorry to hear you were unwell Bill S. Yes the usual stupidity abounds actually this thread is interesting biology meets QM and we flex the science and get to even discuss some science fiction  Talking of that one of the more interesting facts is when the first started doing cryopreserves of people like Walt Disney who paid huge sums on some vein hope that they could be revived in thousands of years. They did not realise that in a mere 5700 years that half the carbon in the body would have decayed so it is going to be much more about genetic advances in being able to clone from partial genetic fragments than having the whole body. The reality was they might as well just frozen a small sample as the entire body. Useless fact of the day 
I believe in "Evil, Bad, Ungodly fantasy science and maths", so I am undoubtedly wrong to you.
|
|
|
|
|
Joined: Dec 2006
Posts: 962
Superstar
|

Superstar
Joined: Dec 2006
Posts: 962 |
Half of the Carbon, or half of the C-14? Ordinarily Carbon is pretty stable. And C-14 decays with the loss of an alpha particle to stable C-12.
If you don't care for reality, just wait a while; another will be along shortly. --A Rose
|
|
|
|
|
Joined: May 2011
Posts: 2,819
Megastar
|

Megastar
Joined: May 2011
Posts: 2,819 |
Yep sorry that english thing again ... the above is indeed misleading now I re-read in that it provides an image of half the body disappearing which is a bit excessive and wrong  I can't stop laughing at the idea of half the body disappearing such a funny thought. However C-14 also decays with beta emmission to N-14 not alpha emission Rose ( http://en.wikipedia.org/wiki/Carbon-14). Thats why we can't do the test in the lab on live subjects ( http://sciencedemonstrations.fas.harvard...gecontent270775) If you look at figure 2 on the side of the wiki link it shows you the balancing act when organisms are alive versus when they die (alive at the top versus dead at the bottom). 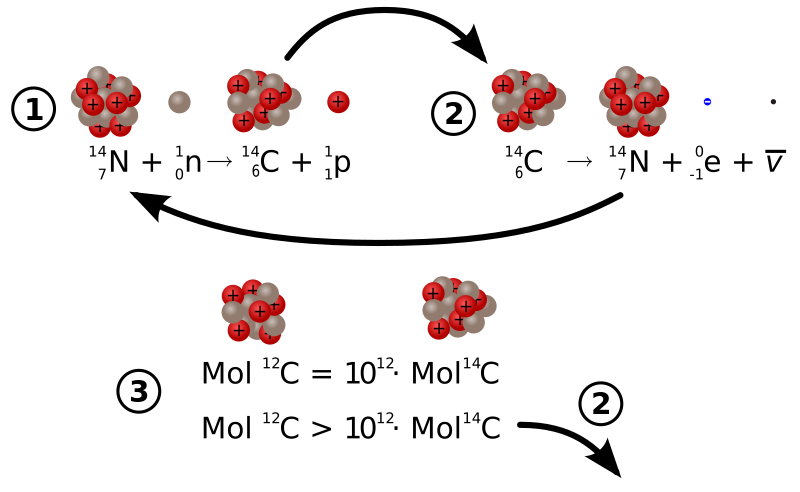 I guess technically what we should say is the half the c-14 bondings which will be (18%(composition of body as carbon) * 1.1% C14 to C-12 ratio)= 0.198% of the body mass will almost certainly unbond when the C14 decays to N14. It will be the same for all unstable isotypes by their decay half lifes and the result will be massive tissue damage. I guess we should also say the cell damage from freezing a body is going to be more massive than the decay damage but at least the chemical compositions and amino acids remain correct from freeze damage where they do not remain correct from decay damage. Mind you this is probably getting way way off track and not relevant to the thread topic. Probably all we need to take out of the whole discussion is carbon based life has an entropic energy overhead to counter the natural radioactive decays. The bigger and more complex the organism the bigger the overhead. None of that is new or controvesal, Ilya Prigogine recieved the Nobel Prize in 1977 for his work in the area blending QM and biology but the work appears not to have made it down to biology. ( http://en.wikipedia.org/wiki/Ilya_Prigogine)
Prigogine notes numerous examples of irreversibility, including diffusion, radioactive decay, solar radiation, weather and the emergence and evolution of life. Like weather systems, organisms are unstable systems existing far from thermodynamic equilibrium. Instability resists standard deterministic explanation. Instead, due to sensitivity to initial conditions, unstable systems can only be explained statistically, that is, in terms of probability.
That better 
Last edited by Orac; 02/28/13 03:42 AM.
I believe in "Evil, Bad, Ungodly fantasy science and maths", so I am undoubtedly wrong to you.
|
|
|
|
|
Joined: May 2010
Posts: 410
Senior Member
|

Senior Member
Joined: May 2010
Posts: 410 |
No person or scientist I know would ever class a Model T more advanced than a modern Ferrari and that is why your argument becomes word play no sensical. But we're not talking about cars here - we're talking about species. And 'advanced' as species go is not as clear-cut as with cars. As I pointed out, so-called 'simple' single-celled organisms can have far more genes (i.e. parts) than us supposedly 'advanced' multicellulars; that also means that they have a theoretically larger capacity to respond to changes in the environment. And 'advanced' or 'higher' is purely relative; which is the main point I was trying to make. A useful (i..e. 'advanced' adaptation in a human may very well be a detrimental feature in another organism. Colour vision is pretty advanced - unless you live 10km below the surface of the ocean... The fact you consider by some word play you can invert and invent a criteria you can reverse that tells you that you are contriving an answer that noone would readily accept. No, I was trying to apply your flawed example of cars to how biology works. Your analogy was false; ergo, it was difficult to make a good go of correcting it. Your logic defies Entropy and in my area Quantum Mechanics your idea is in direct conflict with all it's central tenants. I know QM does not stop because something is alive and as QM has been much more rigorously tested than evolution I can say quite certainly you are wrong. LOL, and you claim I'm scientifically ignorant. Evolution is by far one of the most tested scientific theories out there. Its not called the universal principal of biology for nothing. Think of it this way - physicists are still seeking to understand some very basic aspects of QM; including how probabilistic phenomena at the quantum level translates into the deterministic behaviours we see in the macroworld. In contrast, the TOE is a unified and near-complete theory with great predictive ability. It has withstood over 150 years of scientific enquiry; and while the minutia has changed, the central tenants remain firm. Continuing on.... QM states a system in a highly complex state is probably far from equilibrium and in a low entropy (improbable) state, where the equilibrium state would be simpler, less complex, and higher entropy. Ahh, but herein is two serious problems: 1) With only a few exceptions, living organisms operate on physical principals which are not quantum in nature - the macromolicules of which we are comprised are too large to function in the probabilistic fashion of QM, do not display classical QM behaviours (discreteness, superposition, entanglement, etc), and instead obey the rules of classical physics. Since QM currently lacks a viable and accepted theory of how QM behaviours turn into the sorts of physics we see in the macro-world, it is unclear if you can apply the QM rules to biology - indeed, the general agreement in the field is you cannot. Now before you get excited, let me point out that in a few rare cases quantum phenomena can be found in a small subset of isolated biological macromolciules - to date 3: photon anti-bunching in fluorescent proteins, quantum delocalization is one type of pigment, and a pseudo-quantum computing gate was constructed with nucleotides. The later is obviously a human construct, not an evolved one. There has also been some discussion that olfactory receptors and photosynthesis may function through quantum tunnelling; the evidence there is circumstantial at best, with more conventional ligand-binding (olfactory) and electron-excitation-transport (photosynthesis) models remaining the de facto accepted interpretation at this time. Problem 2, however, is a more serious one. Lets assume that the quantum definition of entropy holds true to the macrolevel. I'm unsure of which QM entropy measure you are referring to - your 'definition' (in quotes as you didn't provide anything that was very clear) doesn't match with any that I'm aware of (which, granted, is limited to Gibbs, von Newman & Shannons as used in information theory); but assuming it is based on similar principals, you ignored two key things: a) it is not purely additive, and b) entropy, in the QM systems I am aware of, increases only as you bias a system towards a subset of possible quantum states. Biologically, the later kills much of your argument - the data we have shows that biological systems do not act at a quantum level, and ergo lack the capacity to drive quantum systems away from equilibrium. Now, perhaps you meant in the context of information theory. But there again, your model fails - we know what information is required to produce living organisms (i.e. genomic contents), and we know there is no correlation between apparently complexity, and genome size/content. That leaves us with classical thermodynamics, which does not stipulate that more complex systems being more prone to failure. You state multicellular is rare and the above explains why if you hadn't realised it before that is the reason. Hydrogen is the most abundant molecule in the universe because it is the most simple stable state same exact reason. Again, your explanation fails. Hydrogen is the most abundant material in the universe for two reasons: 1) It comprised the majority of matter produced during the big bang, and 2) There has been insufficient stellar fusion to alter the balance towards larger atoms. I'd point out that your argument fails at a most basic level - stable isotopes of many more 'complex' atom exist, and they are as stable as hydrogen. Ultimately, the stability of hydrogen or any other stable atoms is determined by the same thing - neutron or proton (if it exists) decay. Granted, H has not neutrons and thus is only limited to proton decay (if it exists). But that said, the half-life of a neutron in a stable atoms nucleus is on the order of 10 15 to 10 44 years - orders of magnitude older than or current universe (10 10 years), meaning that neutron decay is incapable of accounting for the frequency of hydrogen - by many magnitudes of order. I read it - it doesn't support your claim. Its a great article on the anthropic principle, but it in no way, shape or form supports your contention that simple = more stable or lower entropy. Indeed, the term 'entropy' doesn't even appear in the article. It directly refutes your claim that hydrogen is more abundant because it is simpler and thus more stable, and correctly points out that hydrogen is primordial to the big bang, and thus the amount of non-hydrogen compounds is determined by rates of nucleosynthesis in stars. The fallout of the stability decay of all chemicals due to entropic principles means that every atom or molecule in the human body is replaced every 7 to 10 years that is a huge cost to the organism and it is not optional because thats the decay constant of the molecules in the universe the more complex they are the shorter they survive. http://en.wikipedia.org/wiki/Radioactive_decayMan, oh man, did you get that one wrong! It is not radioactive decay that determines the biological half-life of atoms in our bodies; rather it is the continued recycling of biological materials, combined with our continued uptake and loss of material, that produces the effect. For example, the four most common atoms in our bodies are hydrogen, carbon, oxygen and nitrogen. The least stable naturally occurring isotope of these are: H 3: half-life of 12.32 years C 14: half-life of 5730 years N: no naturally existing unstable isotopes O: no naturally existing unstable isotopes So with no turnover, none of these atoms would decay enough to need total replacement in 10 years. It is only because of the continued building and degrading of biological materials that these end up being recycled as frequently as they are. Indeed, the average atom in your body only gets to be in a particular biomolicule (protein, lipid, etc) for a few days days before being recycled into something else. All of the water in your body is cycled every ~20 days...but not because of radiodecay! Even taking the correct view of what is occurring - not radiodecay but rather biological recycling - it doen't support your position that it is 'harder' to be multicellular. Indeed, the recycling rate is inversely proportional to mass - i.e. the smaller you are, the more frequently you completely replace the constituent atoms in your cell/body. As you point out, in humans its about 10 years. In bacteria, its every 2-3 generations; thats as little as 1 hour for some rapidly growing bacteria! Again, a fatal flaw with your argument. These decays occur - but are rare. The vast majority of atom-replacement is simply due to metabolic cycling. But that said, all living organisms will experience the same decay rates, and thus have to replace a proportionate amount of their mass, and suffer a proportionate amount of damage. So if you had a 80kg mass of e coli, they'd experience the exact same burden as a 80kg man. That said, the burden is minute; a thousandth of a percent of the total cycled mass of the organism. Your body is committed to these replacements every second of every day it is alive awake or asleep the entropic principles involved are simply massive. Actually, the radiodecay you mention is a minute burden, compared to the regular entropic processes of our metabolism. But even there, simpler organisms have it harder. Metabolic rates scale inversely with mass, meaning the lowly e coli consumes far more energy, per mass, than a human - and thus disproportionately suffers the energetic and metabolic consequences of this. Moreover, your self-claimed knowledge of entropy should make something quite important very obvious to you - to maintain that elevated metabolic rate means that e coli must continually keep itself farther out of thermodynamic equilibrium than a human (per mass, obviously). After all, the available energy in a system is directly determined by how far out of equilibrium the system is. Indeed, e coli maintains a chemiosmotic potential (energy potential/thermodynamic disequlibrium) of -220mV, while our cells max out at -180mV and average -30 to -60mV. Now, since entropy is a measure of energy in the system, which organism has the larger potential to loose energy? So, contrary to all of your claims: 1) The QM theories of entropy probably don't apply to life, 2) The QM theories of entropy I am aware of (including QM-associated information theory) do not dictate simpler = lower entropy; or indeed, that there is any link between complexity and entropy. 3) Radiodecay is not a significant source of atom turn-over in living organisms, an impacts all organisms equally by mass, and lastly but most importantly, 4) As measured by classical physics, smaller organisms maintain a higher degree of thermodynamic disequilibrium than do larger organisms; i.e. entropy is larger in their lives.
As an aside when people talk about Silicon based life forms these decay rates are the reasons against it. The half life of Carbon-14 is 5700 years the half life of Silicon-32 is a mere 170 years. So if humans were silicon based life forms the entropic overhead is orders of magnitude higher again than a carbon based life form just based on the increased turn over for the organism to stay alive.
Again, completely wrong. Silicon life is considered unlikely for chemical reasons, not radioactive ones. This post is already over-long, so here's a paper. Long story short - it is exceedingly rare in the universe (relative to carbon) and due to it size and bonding characteristics, is incapable of producing the same diversity of molecules as is carbon (and thus is unlikely to be able to support the formation of life-like chemistries). Ultimately everything dies because it loses the battle to this law Wow, I mean wow. This is so wrong I have to double-face-palm myself. Firstly, not everything dies. If this were so, no organism that reproduces by binary fusion would exist. Any such living organism today are merely the current form of an organisms which has lived and replicated continually since the first life arose on earth. At no point in time did the organism die - it divided, it changed, but there is no death between the first cell and todays existing cells. Indeed, for these kinds of organisms the concepts of death and birth don't even work; which of the daughter cells is the mother, which the child? The answer is, of course, 'both and neither'. Death, for non-sexually reproducing organisms = extinction. As for us, you are completely wrong on why we die. It is true that radiodecay of internal atoms does cause some biological issues - namely, it occasionally causes mutation. However, that is not why we die. Why we die is due to a number of factors - mutations are one; but even there, radiodecay of atoms in our bodies account for only ~1:20 mutations. The rest are replication errors or due to chemical damage. Far more involved in our ageing and eventual death are three processes - loss of telomere DNA due to incomplete DNA replication during cell division, mitochondrial exhaustion, and the accumulation of protein & lipid aggregates (these damage our cells). The latter (in theory) could actually be helped by radiodecay - anything that breaks up these aggregates would allow for their clearance. and even the universe itself is not excluded from it. If your selective forces were stronger than QM radioactive decay laws you would have species extinctions on average shorter than a few years and by your own comments its thousands of years. While your QM premise is false, I'm confused by your logic here. Selection need not cause extinction to exist, nor is extinction a measure of the strength of selection. So how you equated the two is a bit of a mystery - as is your conclusion. Your whole argument thus falls to the science axe of Occam's razor. Occam's razor only works if the facts used are true. Since most of your claims were false, so was the inevitable conclusion. Bryan
UAA...CAUGCUAUGAUGGAACGAACAAUUAUGGAA
|
|
|
|
|
Joined: May 2011
Posts: 2,819
Megastar
|

Megastar
Joined: May 2011
Posts: 2,819 |
WOW now things don't die and countless other stupidity I don't know where to begin. No wonder we refer to biological science with derogatory terms and call it soft science and perhaps Platt was right ( http://www.science20.com/rationally_speaking/soft_vs_hard_science_part_i) I did get a good laugh at the rigorous testing perhaps you could tell me the current sigma confidence level you have on evolution? I accept evolution and I found that comical I can only imagine what a creationist like Paul would make of it  Perhaps lets start with Ilya Prigogine and his 1977 Nobel prize perhaps you would like to explain how you know better than everyone else in science and how his work has been overturned. The argument I gave you above is pretty much stock standard his in that there are irrevesible processes such as radioactive decay that put living organisms a hell of a long way from equilibrium. So perhaps you would like to start with explaining how you are overturning Ilya's work on the equilibrium of life.
Last edited by Orac; 03/03/13 10:57 AM.
I believe in "Evil, Bad, Ungodly fantasy science and maths", so I am undoubtedly wrong to you.
|
|
|
|
|
Joined: Feb 2007
Posts: 1,840
Megastar
|

Megastar
Joined: Feb 2007
Posts: 1,840 |
Orac, it's clear from all of the above, even to me, that you have precious little knowledge of biology. Why bring yourself, once more, to the point of having to apologize? There's no justification in calling a reasoned argument 'stupidity', nor for attempting to deride a branch of thorough-going scientific research in which you find yourself in ignorance. Assuming that you consider yourself an expert in some field, take a lesson from history: even the best have made fools of themselves when venturing beyond their own sphere of expertise.
"Time is what prevents everything from happening at once" - John Wheeler
|
|
|
|
|
Joined: Dec 2010
Posts: 1,858
Megastar
|
OP

Megastar
Joined: Dec 2010
Posts: 1,858 |
Rede, I'm not sure what is going on with Orac. He seems to be fairly well versed in QM, but he has a problem with being confrontational. I'm not sure why he is this way. Maybe he just likes arguing, something like Paul.
He seems to be defensive about QM. His take seems to be that QM is the answer to everything, when it is obvious that other simpler explanations work just fine in many cases. Granted that QM does underlie just about everything at the most basic level, it doesn't have to be evoked in areas where applying it produces extremely complex interactions which cannot be calculated.
That includes most areas of biology. Evolution for example is the study of variations in DNA. While DNA is formed from particles which obey QM principles the way it changes can quite easily be followed by much simpler procedures than trying to calculate the QM interactions that produce it.
Bill Gill
C is not the speed of light in a vacuum.
C is the universal speed limit.
|
|
|
|
|
Joined: May 2010
Posts: 410
Senior Member
|

Senior Member
Joined: May 2010
Posts: 410 |
WOW now things don't die and countless other stupidity I don't know where to begin. No wonder we refer to biological science with derogatory terms Insults are expected from some quarters. The biosciences are the darling child of modern scientific research - about 80% of all public science funding goes to it. Given the current funding environment , it is of no surprize that some of the more weak-minded in the low-budget side of things will fall back on inanities like insults to cover up their own inadequacies. Of course, others join the winning team - like the two physicists I collaborate with... I did get a good laugh at the rigorous testing perhaps you could tell me the current sigma confidence level you have on evolution? Depends on which part of the theory you are asking about. The process of evolution is a observed phenomena; ergo its probability is 1.0 without variation - or infinite sigma if you want to measure with SD's. Multi-loci dendrograms (hereditary trees indicating the evolutionary relationship between organisms) are built using bootstrap algorithms, typically using a 3-sigma cutoff for significance. Because we use use concurrence between multiple algorithms to build trees, final sigmas are the range of 6 to 9, which is better than certainty of the Higgs boson. Selection, drift, etc, are quantified using genetic algorithms, using cutoffs of 0.95 or 0.01 (2-3 sigma), but that - of course - indicates the minimal degree of certainty. I accept evolution and I found that comical I can only imagine what a creationist like Paul would make of it  So you find your own ignorance comical...interesting. Perhaps lets start with Ilya Prigogine and his 1977 Nobel prize perhaps you would like to explain how you know better than everyone else in science and how his work has been overturned. I fail to see anything that I wrote which disagrees with his work; indeed, his work is critical to understanding emergent properties - a very process which would be entropically impossible if your interpretation were correct (i.e. that more complex structures are more prone to entropy; the exact opposite of what Prigogine showed with his work on dissipative structures). I'm guessing this is another attempt by you to support your claims by throwing out random bits of science you hope we are not familiar with - like your claims about QM & entropy. Sorry, that doesn't work. I'm quite familiar with the science behind emergent systems, and indeed, use it frequently in my own research...but we'll come back to that later. The argument I gave you above is pretty much stock standard his in that there are irrevesible processes such as radioactive decay that put living organisms a hell of a long way from equilibrium. Actually, your argument is non-stock, as most wouldn't be silly enough to make it. The biological burden of radiodecay is well known - and it is very, very, very small. The impact of radiodecay is a factor of a) the relative abundance of stable vs non-stable isotopes in an organism, and b) the frequency of which these isotopes decay (i.e. their half-life). Abundance is where your argument meets its first death - naturally occurring radioisotopes of the common atoms in living organisms are exceedingly rare - indeed, C 14 is the only one normally found in organisms. The half-lives of tritium, P 32, etc, ensure that they are not found in the environment above trace a amounts. In living organisms, C 14 amounts to ~1 in every trillion carbon atoms. Ergo, even if all the C 14 spontaneously decayed, the impact would be minimal. For example, the 6 billion nucleotides that comprise the DNA content of a single human cell contain ~60 billion carbons (there is ~10 carbons per DNA base). Meaning, if every C 14 spontaneously decayed, you'd introduce damage to a single nucleotide in 1 in every 17 cells. In comparison, DNA replication errors will introduce 200-300 every cell division. Your argument is already dead, but it dies a second death. Not only is the abundance of radioisotopes too low too incur a meaningful fitness burden, but the half-lives of those which get incorporated into our bodies is sufficiently long enough to ensure that the burden is effectively zero. Since C 14 is the only one with measurable abundance, lets continue with it. C 14 has a half-life of 5,730 years. A basic half-life calculation tells us that over a 100-year lifespan (I'm being generous), 1.202% of the C 14 will decay. Meaning that over a 100 year lifetime, radiodecay of C14 will damage one nucleotide in one in every 141 cells. That is a meaningless burden, relative to damage from other sources. So perhaps you would like to start with explaining how you are overturning Ilya's work on the equilibrium of life. I'm not - just as you were wrong about what QM says about entropy in systems, you are also wrong about the implications of Prigogine's work. His work shows the exact, polar opposite of what you claim. Prigogine built on the work of von Forester, who showed that a dynamic system dominated by noise system can encounter what he called an attractor - a set of conditions which are more stable than the other random states the system can occupy. von Foresters models were limited in that they dealt with closed systems; Prigogine extended these works to show that in open systems the influx of new material/energy and the loss of heat (entropy) would allow these attractors to grow, thus forming a stable state well away from thermodynamic equilibrium. The key in that work vis-a-vis life is simple - the state in disequilibrium is *stable* so long as it maintains an open thermodynamic environment. The degree of complexity does not determine the stability (aka, your claim), but rather, the ability to exchange energy & matter with the surrounding environment is what determines the stability. Ergo, more complex organisms are not more unstable simply due to their complexity; rather, their stability is determined by other factors. Bryan
UAA...CAUGCUAUGAUGGAACGAACAAUUAUGGAA
|
|
|
|
|
Joined: May 2011
Posts: 2,819
Megastar
|

Megastar
Joined: May 2011
Posts: 2,819 |
The key in that work vis-a-vis life is simple - the state in disequilibrium is *stable* so long as it maintains an open thermodynamic environment. The degree of complexity does not determine the stability (aka, your claim), but rather, the ability to exchange energy & matter with the surrounding environment is what determines the stability. Ergo, more complex organisms are not more unstable simply due to their complexity; rather, their stability is determined by other factors.
For Bill and Rede I am arguing this because I like many scientists don't accept it. I am sorry that you find that wrong or that there is something going on with me but I am not here to be popular. Bill I take things often back into QM terms because that is where I am most comfortable nothing more nothing less. Here is the background to the argument for your reference ( http://en.wikipedia.org/wiki/Biological_thermodynamics) You will note as discussed in the article Biological thermodynamics is in direct contrast between Ilya Prigogine dissipative systems and Hans Krebs and Alfred Lotka open thermodynamics and later picked up by Albert Lehninger
In 1982, American biochemist Albert Lehninger argued that the "order" produced within cells as they grow and divide is more than compensated for by the "disorder" they create in their surroundings in the course of growth and division. "Living organisms preserve their internal order by taking from their surroundings free energy, in the form of nutrients or sunlight, and returning to their surroundings an equal amount of energy as heat and entropy."
If you look that is exactly the open thermodynamics view that ImagingGeek is pushing. So there is a deep divide in the science here and I find it alarming that as I roll the open entropy discussion back into the QM domain I can falsify it. Entropy for QM has no limits or boundaries it even bought some of the greats like Einstein and Hawkings undone. Johannes Koelman has done what I consider a very good article on Entropy from a layman perspective in his article ( http://www.science20.com/hammock_physicist/what_entropy-89730) I noticed someone has dealt with the obvious issue that if you can not cross the hurdle of entropy the idea is dead in the water a claim I stand by.
In a study titled "Natural selection for least action" published in the Proceedings of The Royal Society A., Ville Kaila and Arto Annila of the University of Helsinki describe how the second law of thermodynamics can be written as an equation of motion to describe evolution, showing how natural selection and the principle of least action can be connected by expressing natural selection in terms of chemical thermodynamics. In this view, evolution explores possible paths to level differences in energy densities and so increase entropy most rapidly. Thus, an organism serves as an energy transfer mechanism, and beneficial mutations allow successive organisms to transfer more energy within their enviroment.
Physics.Org carries a layman reduction of the paper http://phys.org/news137679868.htmlGive me a chance to read the paper and I will see if I am willing to give open thermodynamics a chance. The comic on Johannes Koelman's article says it all your theory run up against the information entropy law then your theory is most certainly wrong. Can I ask if your theory is compatable with John Avery Scales version Evolution and QM Information it will make terms alot easier ( http://en.wikipedia.org/wiki/John_Scales_Avery)
The apparent paradox between the second law of thermodynamics and the high degree of order and complexity produced by living systems, according to Avery, has its resolution "in the information content of the Gibbs free energy that enters the biosphere from outside sources"
To the rest of you please note:
Entropy and the origin of life
The second law of thermodynamics applied on the origin of life is a far more complicated issue than the further development of life, since there is no "standard model" of how the first biological lifeforms emerged; only a number of competing hypotheses. The problem is discussed within the area of abiogenesis, implying gradual pre-Darwinian chemical evolution. In 1924, Alexander Oparin suggested that sufficient energy was provided in a primordial soup. The Belgian scientist Ilya Prigogine was awarded with a Nobel prize for an analysis in this area. A related topic is the probability that life would emerge, which has been discussed in several studies, for example by Russell Doolittle.[12]
A reasonable discussion on http://www.eoht.info/page/Thermodynamics+of+Evolution
Curiously, to note, in laying out this argument they choose to utilize Helmholtz free energy (constant volume processes) rather than Gibbs free energy (constant pressure processes), which can explain the formation of biological structures. It is likely that Prigogine chooses his presentation in this manner so as to not effect a weakening in his later arguments; in the sense that he wants to discredit any detail not in alignment with his view that nonequilibrium thermodynamics is the key to explain biological evolution.
So even in 1972 there was two directly competing theories. You may also like to read http://www.eoht.info/page/Human+thermodynamics take note of the modern perspectives section. So I fail to see how I can be called argumentative just because I don't accept ImagingGeeks very singular view. I am condescending of him because he thinks Entropy is something that doesn't matter. QM bought greats like Hawking (Black hole information paradox) and Einstein (Einstein model of a solid) theories down with it do you think we are going to let biology get a freee ride. What I finding interesting is the certainty ImagingGeek portraits in his theory and you all go along for the ride. I don't even claim that with the Big Bang, Universe expansion or even the discovery of the Higgs. Normal sane scientists discuss openly the possibilities of conflicting theories but apparently the thermodynamics of evolution of life only has one theory according to all you people and the science is settled so perhaps you can show me the proof  For a comparisson here was the latest from Jester ( http://resonaances.blogspot.com.au/2013/02/when-shall-we-call-it-higgs.html). You will note the H-Word is still banned at CERN probably because they are hard scientists and actually care that they get the truth.
Last edited by Orac; 03/04/13 07:47 AM.
I believe in "Evil, Bad, Ungodly fantasy science and maths", so I am undoubtedly wrong to you.
|
|
|
|
|
Joined: May 2010
Posts: 410
Senior Member
|

Senior Member
Joined: May 2010
Posts: 410 |
For the sake of brevity, I've clipped most of oracs post. I would just like to add that most of the articles he linked to are excellent ones - but IMO, none of them support his contention that more complex = more susceptible to damage/less adaptable. I'll only hit on a few key points. I just want to interject here by saying I think Orac has misunderstood some of the points I have made - indeed, he seems to be arguing for the same point of view in several places. However, I would at this point also mention that he is mixing up two distinct processes - one of which I suspect he & I agree upon completely, and one which he is arguing against (incorrectly, IMO). Biology is dominated by two thermodynamic processes - those which require energy in the form of things like ATP or NADP/NADPH and those which rely on other processes such as emergence. The former follow the typical 'rules' of thermodynamics, while the latter are driven by processes such Ilya Prigogine's (Ilya from now on, for simplicity) dissipative systems (i.e. emergent systems). These are processes like the formation of some cellular structures, protein folding, membrane formation, etc. These do not consume cellular energy sources, and yet create order that seems to disobey classical thermodynamics. Orac seems to be arguing that only those later processes drive the thermodynamics of living organisms - if so (and my apologies if I have mis-interpreted), then in a word, he is wrong. But, if someone were to argue only the former were involved, they too would be wrong. You need both for life. Emergent properties are critical for life, and they obey the 'rules' discovered by Ilya et al. But they cannot persist for any length of time without the continued manufacture of the base materials (proteins, lipids, etc), and the continued removal of 'poisons' (damaged/misfolded proteins, oxidized lipids, etc) - and these are all energy-dependent processes that are powered by chemiosmotic energy-generating pathways that obey classical thermodynamics. If you look that is exactly the open thermodynamics view that ImagingGeek is pushing. That is not, nor has ever been, my position. Please read what I wrote before putting words in my mouth. So there is a deep divide in the science here and I find it alarming that as I roll the open entropy discussion back into the QM domain I can falsify it. But for 2 major issue: 1) as far as we can tell, you've not accurately described what QM says about thermodynamics - indeed, what you've claimed runs contrary to Gibbs, Ilya, etc. 2) you (and AFAIK, all physicists) have yet to demonstrate that QM processes operate at any measurable level at the macromolecular level of biology. Ergo, you've falsified nothing. I noticed someone has dealt with the obvious issue that if you can not cross the hurdle of entropy the idea is dead in the water a claim I stand by.
...In this view, evolution explores possible paths to level differences in energy densities and so increase entropy most rapidly.
Firstly, I fail to see how this supports your argument - that thermodynamically favoured processes would offer a selective advantage is given - however, it does not support your contention that more complex = higher susceptibility to damage/failure. Secondly, while the article is interesting, it makes one fundamental flaw (and one commonly seen when physicists try to do biology) - the authors incorrectly assume that all evolution is adaptive. Most evolution is not - indeed, some (sexual) tends towards maladaptive; most (95% or more) is neutral. Ergo, the bulk of evolution should occur with a neutral or even negative entropic effect. So I fail to see how I can be called argumentative just because I don't accept ImagingGeeks very singular view. I'd just like to point out that I would consider 'argumentive' a complement - not accepting things at face value is a good characteristic of a scientist. However, given that you don't seem to understand what I ahve written (nor provided any evidence to disprove my 'disproofs' of your claims), I'd say you're still a ways away from a real scientific argument in favour of your position. I am condescending of him because he thinks Entropy is something that doesn't matter. You would have had to completely ignore everything I wrote to conclude that. I guess we can chalk your condescention upto compensation for poor reading comprehension. What I finding interesting is the certainty ImagingGeek portraits in his theory and you all go along for the ride. I don't even claim that with the Big Bang, Universe expansion or even the discovery of the Higgs. Normal sane scientists discuss openly the possibilities of conflicting theories but apparently the thermodynamics of evolution of life only has one theory according to all you people and the science is settled so perhaps you can show me the proof  Actually, Orac, I've given you multiple opportunities to discuss the thermodynamics of evolution - but you've consistently failed to provide any support for your arguments, nor any counter-evidence when I point to data that disproves your theory. A key part of any scientific discourse is evidence. To date, you've provided none; the best you've given are a handful of papers which are either unrelated or which run counter to your claims. Moreover, you've ignored and/or failed to respond to every point & paper I've posted. You cannot be surprised that given those actions on your part, that we treat you as a troll rather than as a serious participant in the thread. Bryan EDIT: I forgot to add one thing. Abiogenesis an evolution are two completely separate processes, dominated by very different energetics and processes. One cannot make conclusions of one based on the other. Indeed, until an abiogenic process becomes self-replicating it is intrinsically incapable of undergoing anything even resembling evolution.
Last edited by ImagingGeek; 03/04/13 11:05 PM.
UAA...CAUGCUAUGAUGGAACGAACAAUUAUGGAA
|
|
|
|
|
Joined: May 2011
Posts: 2,819
Megastar
|

Megastar
Joined: May 2011
Posts: 2,819 |
My problems are so deep and fundemental I have stopped even bothering to try and argue my argument ImagingGeek. What I am trying to do is to fill in the chasms of science between us. I am ignoring my argument not because I think it's wrong but because there are some basics here that are so flawed it is like arguing with a creationist or Paul. Until you understand the science flaws and problems and we address them arguing the actual description is pointless. Now you may argue I have similar flaws on biology and my biology is limited so there is indeed some confusion between us but I think much of it is the chasm of science betweeen us. I am not backing away from any of my statements and we will hopefully get back to each and every one if you hang in. My problems from your above post and I will deal with them in order of science concern for me 1.)Entropy
But for 2 major issue:
1) as far as we can tell, you've not accurately described what QM says about thermodynamics - indeed, what you've claimed runs contrary to Gibbs, Ilya, etc.
Hmmm where to even start with this mess and lets see if I can do it at a layman level. Note I am not even going to bother with my argument at the moment I literally want to fill in some of the gaps Please read carefully the article( http://www.eoht.info/page/Thermodynamics+of+Evolution) There are two sorts of entropy systems discussed being Helmholtz free energy and Gibbs free energy. Ilya Prigogine chose Helmholtz free energy. Now if you read the link ( http://www.eoht.info/page/Helmholtz+free+energy) The key point is "The Helmholtz free energy is commonly used in experiments such as in explosives research (where explosive reactions by their nature induce pressure changes)."So what Ilya is doing is literally equating life as an explosion. So if we take that out the more complex an organism the further from equilibrium it is and the more pressure it took to get it there. That is about as layman as I can write it and we can expand it from there and discuss implications as we need.
2) you (and AFAIK, all physicists) have yet to demonstrate that QM processes operate at any measurable level at the macromolecular level of biology.
And that is where I just start rolling my eyes and realise how little you know of QM. The atoms in your biology is based on QM, the reactions and there rate are controlled by QM and as far as we know all energy in every system ever studied from black holes and suns to galaxies and the universe can be defined by it's equations. Every process in your biology could be described by QM it's just a little more complex for the actually biologists to learn. Some of your people like John Scales Avery are trying perhaps I could recommend you try reading his book "Information Theory and Evolution". I would also point out that when you biologists actually bother to start looking for QM effects funny how you find them The initial discovery of QM in photosynthesis http://physicsworld.com/cws/article/news/2010/feb/04/quantum-mechanics-boosts-photosynthesisThe follow up checking http://phys.org/news/2012-01-role-quantum-effects-photosynthesis.htmlThat I believe falsifies your statement from above at even the most crass level. At a more advanced level in your layman terms QM is acting like a catalyst and that should at least get you questioning what else it may be involved in. If it pricked your interest nature did a good article and note the first sentence( http://www.nature.com/nphys/journal/v9/n1/full/nphys2474.html) At the most advanced level layman often confuse QM not realising it is simply a description of the universe as we know it. QM itself is responsible for no effect and can not be the cause of anything, you need a theory of everything for that. The fact we could describe life under QM theory would simply imply it is like everything else in the universe and that alone may upset some creationists. So QM is not responsible for evolution but it may provide a useful tool to look at the effect a thought that might interest you. It is true I see QM everywhere as Bill claimed but that is simply because I haven't found anything not in the universe yet. If you have any questions please ask but basically any sane hard scientist is not expecting life to be some how magically exempt from the description and laws of QM. 2.)Drivers of evolutionIt appears you are still claiming there is only one theory for the driver of evolution can I please get a link or description to that so I can read and study it. 3.)Types of evolutionFrom the above you seem to be seperating out different types of evolution micro,macro etc and in your final added comment you are now even seperating creation of life (Abiogenesis). I find the last seperation interesting in that by your own discussion you can't have evolution until things can replicate and you even claim they are driven by different energetics. Could you elaborate on your theory of how this all works? I read the section on wikipedia on ( http://en.wikipedia.org/wiki/Abiogenesis) and I myself would favour John Desmond Bernal views. Note this is my personal view and I am not claiming it is right but I can think of a very compelling argument which when I get you across the entropy landmine we can discuss.
Stage 1: The origin of biological monomers
Stage 2: The origin of biological polymers
Stage 3: The evolution from molecules to cell
Bernal suggested that evolution commenced between Stage 1 and 2
BTW if your two physicists you say work with you are worth a crumpet ask them why I may take such a view. Anyhow clearly I am wrong according to you so lets first deal with the great biology view of it all.
Last edited by Orac; 03/05/13 04:36 AM.
I believe in "Evil, Bad, Ungodly fantasy science and maths", so I am undoubtedly wrong to you.
|
|
|
|
|
Joined: May 2011
Posts: 2,819
Megastar
|

Megastar
Joined: May 2011
Posts: 2,819 |
At this point I am adding in an amusing QM story that has sort of been playing out and creating much humour for me. In 2010 to much noise this article appeared on Quantum Darwinism http://phys.org/news192693808.htmlThe New Journal of Physics carried a new expanded version last year http://iopscience.iop.org/1367-2630/14/8/083010/articleSome even claimed it was a new bright wonderful theory unifying evolution and quantum mechanics. What do I make of it .... the claims are a pile of rubbish and thats being kind !!!!! Repeating from the post above QM drives absolutely NOTHING it is simply a description of some underlying physics process some of which are and some of which are not understood. Given that QM as best we know describes the entire universe it should be hardly surprising you can extend the description to cover living organisms something I have actually been arguing with you. So congratulations to my fellow QM scientists they discovered what they should have known from the start and it should have been no surprise that QM can describe what is happening in evolution. To be honest I would have been much more surprised and shocked if they showed the reverse. The bottom line is this garbage takes you no closer to understanding what is driving evolution it simply shows QM can describe it ... and that ends the memo from this.
Last edited by Orac; 03/05/13 01:31 PM.
I believe in "Evil, Bad, Ungodly fantasy science and maths", so I am undoubtedly wrong to you.
|
|
|
|
|
Joined: May 2010
Posts: 410
Senior Member
|

Senior Member
Joined: May 2010
Posts: 410 |
So what Ilya is doing is literally equating life as an explosion. So if we take that out the more complex an organism the further from equilibrium it is and the more pressure it took to get it there.
That is about as layman as I can write it and we can expand it from there and discuss implications as we need. Firstly, lets be clear - I don't need the layman's description. In my lab we study (among other things) the spontaneous formation of membrane microdomains, using GP Gladyshev's model of Prigoginean thermodynamics as our analytical basis. So instead of the lame (and factually incorrect) laymans descriptions, lets actually talk about the science. Secondly, you've not dealt with the problems that your original claims vis-a-vis QM entropy are still wrong, and the new article you does not support your contention that higher complexity = farther form thermodynamic equilibrium. Finally, you've completely mis-represented (i.e. lied) about what Prigoginean thermodynamics says about biological systems. It says nothing relating to complexity. What it actually says is the further something is from thermodynamic equilibrium the more pressure there *should* be to return to equilibrium. Since this doesn't happen with life, you need to invoke processes such dissipative structures to explain the apparent stability off of thermodynamic equilibrium. These are what allow you to maintain a state of non-equilibrium (i.e. life), through dissipating entropy to the external environment.
2) you (and AFAIK, all physicists) have yet to demonstrate that QM processes operate at any measurable level at the macromolecular level of biology.
And that is where I just start rolling my eyes and realise how little you know of QM. The atoms in your biology is based on QM, the reactions and there rate are controlled by QM and as far as we know all energy in every system ever studied from black holes and suns to galaxies and the universe can be defined by it's equations. If you're rolling your eyes, than your grasp of QM is poorer than mine. QM processes such as superposition, entaglment, quntatization, etc, are not observed in macromolicules; indeed, atoms quite often don't exhibit these behaviours. How to explain the loss of quantum coherence in larger structures, and how group behaviours lead to decoherance, has been for over 50 years the single greatest unanswered question in QM. We have found a whopping total of 1 - in a system evolved to efficiently capture photons. It is, and remains, the only such system known. And its arguable if coherence explains it - the counter argument has been forster energy transfer, which is as explanative an explanation. You've presented one half of an ongoing debate and claimed it as 'proof'. That is quite dishonest. And, I'd point out, that showing one biological structure that has evolved to pass on electron excitation energy is a far cry from demonstrating that QM processes involved in QM models of entropy - coherence, entanglement, etc - have measurable impacts on biological polymers. It appears you are still claiming there is only one theory for the driver of evolution can I please get a link or description to that so I can read and study it. So you're arguing against evolutionary theory without being familiar with it? A few books that cover the basics: The Greatest Show on Earth: The Evidence for Evolution by Richard Dawkins The Tangled Bank: An Introduction to Evolution by Carl Zimmer There are a lot more, but those are two of my favourite "introductory"-type books. They give a pretty good summation of the basic theory, but do not go into the more technical aspects such as quantifying selection, etc. 3.)Types of evolution
From the above you seem to be seperating out different types of evolution micro,macro etc and in your final added comment you are now even seperating creation of life (Abiogenesis). I never used the term 'types of evolution', so I have no idea what you are talking about. I did discuss the outcomes of evolution - i.e. adaptive vs. neutral vs. maladaptive. I also discussed positive vs negative selection. But those are all evolution, not different kinds. I would never separate macro from micro - they are simply different quantities of the same thing. All evolutionary biologists will tell you that abiogenesis is a separate science, driven by different processes, than evolution. Confounding the two is a common creationist tactic. I'd direct you to the above two books for an explanation of the issue. I find the last seperation interesting in that by your own discussion you can't have evolution until things can replicate and you even claim they are driven by different energetics.
Could you elaborate on your theory of how this all works? I should refine my answer by saying "potentially driven by other energetics". Replication is a pre-requisite for evolution, as by definition, evolution is 'descent with modification'. If you cannot replicate - and replicate imperfectly, you cannot evolve. As for elaboration, it is simple. Abiogenesis involves three processes: 1) the formation of biopolymers (protein, RNA, DNA, lipids, etc), 2) the formation of energy-generating processes (i.e. metabolism) and 3) the combination of 1 & 2 into a self-replicating system. The formation of monomers and polymers happens spontaneously, following classical chemical thermodynamics - things react, you end up with products with lower free energy, Gibbs is happy. However, the formation of these into complexes & structures doesn't necessarily obey Gibbs free energy or use conventional biological mechanisms (due to a lack of metabolism); meaning that these higher-order structures rely on emergent properties - i.e. Prigogine's dissipative structures.
Stage 1: The origin of biological monomers
Stage 2: The origin of biological polymers
Stage 3: The evolution from molecules to cell
Bernal suggested that evolution commenced between Stage 1 and 2
And I would agree - sort of. One of the more accepted models of abiogenesis is the RNA world model; essentially, RNA (which forms spontaniously under abiotic conditions) polymerizes, a small portion of those are (by chance) ribozymes capable of self-replication, and thus can 'evolve'. However, this 'evolution' is limited to the ribozymes; it will not 'extend' to associated molecules or metabolic pathways - i.e. there is no phenotype or selective advantage at the level of any proto-organism. The formation and incorporation of non-ribozymes would still be a simple stoichastic process not involving replication, modification or inheritance. More biologists place the beginning of evolution at the proto-cell stage; the step where metabolic processes are also genetically encoded, encapsulated in a cell-like structure, and thus there is a pseudo-organism capable of inheritance, modification and phenotypes. Anyhow clearly I am wrong according to you so lets first deal with the great biology view of it all. But you're wrong about (or deliberatly misrepresenting) the physics as well, so we're still at zero... Bryan
UAA...CAUGCUAUGAUGGAACGAACAAUUAUGGAA
|
|
|
|
|
Joined: May 2011
Posts: 2,819
Megastar
|

Megastar
Joined: May 2011
Posts: 2,819 |
Secondly, you've not dealt with the problems that your original claims vis-a-vis QM entropy are still wrong, and the new article you does not support your contention that higher complexity = farther form thermodynamic equilibrium.
Finally, you've completely mis-represented (i.e. lied) about what Prigoginean thermodynamics says about biological systems. It says nothing relating to complexity. What it actually says is the further something is from thermodynamic equilibrium the more pressure there *should* be to return to equilibrium. Since this doesn't happen with life, you need to invoke processes such dissipative structures to explain the apparent stability off of thermodynamic equilibrium. These are what allow you to maintain a state of non-equilibrium (i.e. life), through dissipating entropy to the external environment.
As I said I am not forgetting this arguement at all and I want to come back to it. I totally disagree with everything you have written but it can wait for now I need to fill in some detail. It is you who is now being argumentative I have said on at least 3 occasions lets sort the science and come back to this.
And that is where I just start rolling my eyes and realise how little you know of QM.
If you're rolling your eyes, than your grasp of QM is poorer than mine. QM processes such as superposition, entaglment, quntatization, etc, are not observed in macromolicules; indeed, atoms quite often don't exhibit these behaviours. How to explain the loss of quantum coherence in larger structures, and how group behaviours lead to decoherance, has been for over 50 years the single greatest unanswered question in QM.
Sorry you make me laugh with that statement ... who told you all that rubbish. Lets deal with the statements one by one because it is important
QM processes such as superposition, entaglment, quntatization, etc, are not observed in macromolicules; indeed, atoms quite often don't exhibit these behaviours.
Perhaps start with the grand daddy of this area Anton Zeilinger ( http://en.wikipedia.org/wiki/Anton_Zeilinger)
In 1999, Zeilinger abandoned atom optics for experiments with very complex and massive macro-molecules - fullerenes. The successful demonstration of quantum interference for and molecules (fullerenes) in 1999 opened up a very active field of research. Key results include the most precise quantitative study to date of decoherence by thermal radiation and by atomic collisions and the first quantum interference of complex biological macro-molecules. This work is continued by Markus Arndt.
You may care to read Markus Arndt's discussion paper. http://www.uam.es/personal_pdi/ciencias/jcuevas/Teaching/double-slit-C60.pdfThere work has continued and there last paper in 2012 was published in Nature http://www.nature.com/nnano/journal/v7/n5/full/nnano.2012.34.htmlThe original work was extended into normal objects in 2010 in a blaze of glory they entangled the first macro object http://physicsworld.com/cws/article/news/2010/mar/18/quantum-effect-spotted-in-a-visible-objectIn 2011 it was etended into an aluminum structure http://www.nist.gov/pml/div686/drum-070611.cfmAnd in december 2011 we saw two diamonds put into entanglement http://www.nature.com/news/entangled-diamonds-vibrate-together-1.9532In all of the above the systems were entangled but as you pointed out that is only one sort of quantum feature so the others were put to the test to see if we have Quantum correlations without entanglement and that works too. http://phys.org/news/2011-08-quantum-entanglement.htmlhttp://nextbigfuture.com/2012/08/quantum-technologies-soon-quantum.htmlThis year the first quantum refrigerator was produced by getting a large platten area to behave in a quantum manner. http://phys.org/news/2013-03-quantum-refrigerator-extreme-cooling-convenience.htmlThe bottom line here is all molecules, atoms and objects have intrinsic QM properties all of those discoveries come about because of QM information theory and proof of one of it's tennants being Quantum Discord ( http://en.wikipedia.org/wiki/Quantum_discord) In other words every atom, every molecule in the universe is locked in some sort of quantum mixed-state it is simply our ability to see it as an observer that is lacking. So now lets deal with the last part of your statement
How to explain the loss of quantum coherence in larger structures, and how group behaviours lead to decoherance, has been for over 50 years the single greatest unanswered question in QM.
So what your think is a 50 year old question has been answered in the last 10 it doesn't totally decohere it moves it a state of quantum discord between the normal macro world and the quantum world. And in wikipedia under quantum decoherence since you are as you say very scientific you will find it explained thus
Decoherence does not generate actual wave function collapse. It only provides an explanation for the observance of wave function collapse, as the quantum nature of the system "leaks" into the environment. That is, components of the wavefunction are decoupled from a coherent system, and acquire phases from their immediate surroundings. A total superposition of the global or universal wavefunction still exists (and remains coherent at the global level), but its ultimate fate remains an interpretational issue. Specifically, decoherence does not attempt to explain the measurement problem. Rather, decoherence provides an explanation for the transition of the system to a mixture of states that seem to correspond to those states observers perceive. Moreover, our observation tells us that this mixture looks like a proper quantum ensemble in a measurement situation, as we observe that measurements lead to the "realization" of precisely one state in the "ensemble".
The important answering your question is this => A total superposition of the global or universal wavefunction still exists (and remains coherent at the global level), but its ultimate fate remains an interpretational issue. Got it the global coherence NEVER DIES OR STOPS that we know it is sort of like the cosmic background radiation in laymans terms and your 50 year old mystery is solved for you. So everything in that statement is factually wrong under modern QM we know what decoherence is and does which has been proven by countless testing and why I am certain that life should be able to be completely described in QM terms.Do you want to argue further on the above issue or do you accept I do know a little about modern QM and what I am telling you is correct and proven versus your circa 1970 version of QM?
And, I'd point out, that showing one biological structure that has evolved to pass on electron excitation energy is a far cry from demonstrating that QM processes involved in QM models of entropy - coherence, entanglement, etc - have measurable impacts on biological polymers.
Again I will point out QM is doing nothing it is a description of the universe and that description as far as we know holds for anything in the universe. Whatever the system QM is describing is some underlying theory and it is that which controls the rules of the universe and I am sorry all chemistry must obey those rules a fact readily accepted by most chemists. What you are arguing is that biological polymers are now exempt from rules that as far as we know every other thing in the universe plays by based on what?
I would never separate macro from micro - they are simply different quantities of the same thing. All evolutionary biologists will tell you that abiogenesis is a separate science, driven by different processes, than evolution. Confounding the two is a common creationist tactic. I'd direct you to the above two books for an explanation of the issue.
Thats fine we are in agreement on that fact because at a QM level I can expalin to you why there ultimately must break down to a subset that involves only one driving factor, we call it emergent behaviour.
I should refine my answer by saying "potentially driven by other energetics". Replication is a pre-requisite for evolution, as by definition, evolution is 'descent with modification'. If you cannot replicate - and replicate imperfectly, you cannot evolve. As for elaboration, it is simple.
<... snip ....>
Again in QM we also deal with emergent behaviour and two energetics and drivers both stable and the second able to piggy back off the first well lets just say it's problematic and you might as well invoke GOD at that point. It is why we don't think about gravity for example as first being something other than what we see now that was created by some initial event and then suddenly some other effect piggy backing off the back of it to become what it is now. Good luck holding the universe together thru that change. So I would say your refined answer is the better one and why I said to ask your physicists. So I would be in agreement with your refined answer. In QM speak things tend to explosively change between meta-stable mixed-states on large QM systems, you would literally get an explosion of life  See some answers require actually no knowledge of biology itself something that may perhaps surprise you. Topically Ethan Siegel did an article on the journey of one atom ( http://scienceblogs.com/startswithabang/2013/03/06/an-atom-in-the-universe/) and I would argue we can track that entire journey and all the interactions along the way using QM to describe it  If you are happy with all that we may be ready to discuss simple versus complex life energetics.
Last edited by Orac; 03/07/13 04:35 AM.
I believe in "Evil, Bad, Ungodly fantasy science and maths", so I am undoubtedly wrong to you.
|
|
|
|
|
Joined: Jun 2008
Posts: 1,249
Megastar
|

Megastar
Joined: Jun 2008
Posts: 1,249 |
I was addicted to the Hokey Pokey, but then I turned myself around!!
|
|
|
|
|




